An Interview with Dr. Zhixing Chen
Posted by Subhajit Dutta, on 19 June 2024
Welcome to the FocalPlane Interview Series where we spotlight groundbreaking Asian-origin scientists pushing the boundaries of cell biology and microscopy. Today, we’re delighted to feature Dr. Zhixing Chen from the Peking University, China. In this exclusive interview, Dr. Chen unveils his transformative research on probe design, shares insights into his remarkable career path, and offers a glimpse into the exciting possibilities for synthetic chemistry in this field.

Bio in Brief: Dr. Zhixing Chen is a Principal Investigator and Assistant Professor at Peking University (China), leading a dynamic research group dedicated to revolutionising bioimaging through the development of novel molecular probes. With a strong background in chemistry and extensive experience in organic synthesis, polymer chemistry, and live-cell imaging, Dr. Chen is committed to harnessing the power of chemistry to drive breakthroughs in molecular imaging and molecular medicine. Dr. Chen’s lab focuses on designing “tailored molecules” to address critical challenges in 4D live cell bioimaging. By integrating chemical synthesis, spectroscopy, and chemical biology, his team creates cutting-edge probes and sensors that enable deeper insights into cellular processes and disease mechanisms.
Q. How did you start your career in science, particularly in novel probe design for bioimaging?
A. I began as an organic chemist, drawn to the ‘designer’ aspect of chemistry. Our lab works on molecular engineering – building tools for science. I really like this mindset. This resonates deeply with my approach to research. I started my PhD at Columbia University in New York in 2009, which was just after Professor Martin Chalfie was awarded the Nobel Prize (2008) for introducing GFP as a fluorescent biomarker. Professor Virginia Cornish at Columbia had a project aiming to combine the brightness of synthetic dyes with the genetic inclusivity of fluorescent proteins using TMPTag. So, I decided to join her lab and work in the field of labelling technology. This project introduced me to the field of bioimaging.
Q. Are there any past and current scientists whom you admire and who inspired your research?
A. Roger Tsien is my primary role model. His work on calcium indicators showcases the best combination of chemistry principles, from photochemistry to inorganic and organic chemistry, and biological applications in neuroscience and cell biology.
Q. What are the major challenges in bioimaging that need to be resolved by designing new probes?
A. The biggest challenge is identifying the most important problems to solve. Chemists can solve many problems, but knowing which ones are truly significant is crucial. And, it is generally true in any interdisciplinary lab, if I have a good hammer, I need to find really the most important nail that I can fix it.
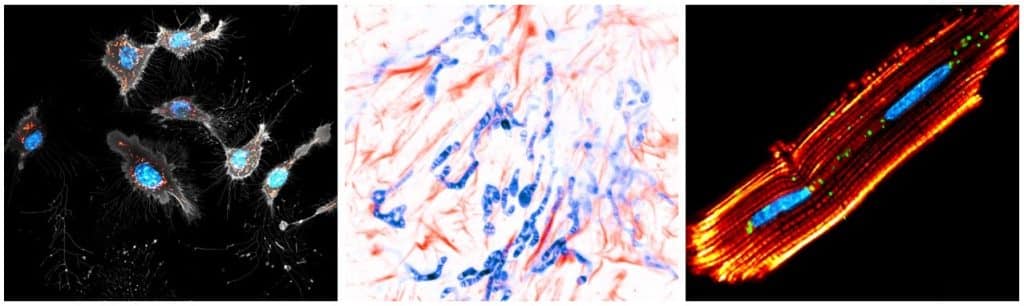
In my lab, we’ve recognized a gap in mitochondrial imaging. Despite existing tools, there’s still a need for super-resolution imaging compatible probes. This led to the development of the PKmito probe, a simple chemical approach with a significant impact on cell biology and microscopy. I think from a probe side, from a microscopy side, and from a cell biology side, there are all different perspectives, which are important to consider. So, I think the biggest challenge that I’m facing is trying to find a common interest between the three parties, and also something that I’m capable of doing.
Q. What are your future research goals?
A. In the next 5-10 years, our focus will be on upgrading mitochondrial probes of different colours for super-resolution STED microscopy while minimizing phototoxicity. The aim is to enable routine 4D super-resolution imaging for cell biology.
Q. Why do some organelles, like mitochondria, or some ions, like calcium, have more probes but many others have fewer or none?
A. It’s challenging to design probes for many targets with small molecules. Success is often due to luck, while failure is the norm. Mitochondria, for instance, are relatively easy to target because they’re the most negatively charged areas inside cells. This allows hydrophobic, positively charged molecules to accumulate there. Other organelles are much harder to target.
Most frequently, for example, if you want to image DNA, the way to do it is to conjugate a dye to a DNA binder. Sometimes the dye itself is the DNA binder because a lot of dyes are positively charged, and the nucleic acids are negatively charged. You can play with this charge trick on selected organelles, but if you want to visualize something like actin or tubulin, it’s much harder.
Q. Can you share the story of your new probe?
A. In 2018, I joined Peking University’s College of Future Technology, drawn to its interdisciplinary environment. It was there that I realized mitochondrial imaging was lagging behind other areas. Through conversations with my colleague, microscopist Dr. Liangyi Chen, I learned about the pressing issue of phototoxicity in mitochondrial imaging, especially with super-resolution techniques.
This spurred me to develop a gentler alternative to existing mitochondrial trackers. We drew inspiration from earlier work on triplet-state quenchers, which had primarily been used in single-molecule biophysics. We realized that applying this concept to live-cell imaging maybe even more impactful due to the phototoxicity challenges in that context. We repurposed what the field already had, and these probes are bright, positively charged and therefore suitable for mitochondria. With Dr. Liangyi Chen, we demonstrated this under the microscope and published our first paper. In 2021, we tried our mitochondrial dye candidates with a STED demo machine at Peking University. Using our image, Abberior Instruments has posted the images on social media to advertise their microscope and that got many people`s attention. Following that we made a collaboration with Dr. Stefan Jakobs, from Max Planck Institute for Multidisciplinary Sciences, Göttingen. They helped us taking a lot of beautiful movies and that’s why we published a paper together with the Jakobs lab as a cover story on PNAS. Yeah, that’s basically the story. But what I want to say is, that it’s really my colleagues, Dr. Liangyi Chen and Dr. Stephen Jakobs, who taught me how important this problem is. I’m happy to see that the story unfolds in such a way.
Q. Can you tell me about your group? What has been your philosophy in training and mentoring young scientists?
A. My lab at Peking University’s College of Future Technology is truly interdisciplinary, with half the members from chemistry backgrounds and the other half from biology. Managing such a diverse group is challenging, as they often speak different scientific languages. My goal is to ensure everyone understands each other, receives comprehensive training, and grasps the big picture.

One of my biggest dilemmas is balancing depth with breadth in training. Should students focus on one area deeply or gain a diverse skillset? I worry about them spreading themselves too thin or becoming too specialized for interdisciplinary work.
I faced a similar dilemma during my PhD, training in organic chemistry, chemical biology, spectroscopy, and microscopy. This left me questioning my identity as a scientist. While I still don’t have a perfect answer, I’ve adopted a philosophy of providing students with maximum freedom to explore and develop their interests within our diverse and interactive environment. Eventually, some of my students delve into protein engineering, others into synthetic chemistry or cell biology – a wide range of different directions.
Q. How is your life at Peking University? Your institute has a very interesting name, I must say, College of Future Technology; what is the story behind that?
A. Regarding our research home, I initially found the name “College of Future Technology” embarrassingly grandiose. However, it’s grown on me. It encourages us to adopt a futuristic mindset, much like Elon Musk’s approach to technology development. Instead of chasing trendy topics, we consider what the future of bioimaging will look like and what’s hindering us from getting there. Cell biology research is shifting from static images to dynamic videos, mirroring the trend seen on social media where people used to post pictures and now they are posting videos more. This shift is driving a growing demand for time-lapse and live-cell super-resolution imaging, which is expected to be the future of cell biology. This top-down thinking led us to focus on solving the phototoxicity problem, which has been largely overlooked but is crucial for the future of 4D live-cell imaging.
China’s investment in research and Peking University’s dynamic approach to emerging technologies have created a unique opportunity. The university’s emphasis on bioimaging led to the establishment of the National Biomedical Imaging Center, where I’ve had the chance to collaborate with diverse scientists from interdisciplinary backgrounds. This international exposure at our university has been invaluable, fostering new partnerships and exciting research directions.
Q. Being so successful requires a lot of work hours, especially in China. How do you cope with it? Do you have any interests outside the lab?
A. I think in China, the country runs itself at a very fast pace, making it really competitive and vibrant. That means we have really little life. I play badminton and soccer, but I found myself constantly travelling this year with little leisure time for sports. I have a three-year-old daughter and my family spent quite some time together. Yeah, it has been good. But life here is really fast-paced!
Q. How do you think cell biology and microscopy are evolving as a domain? At the present time, AI is evolving a lot and it seems like the field is going through a quick transition phase. What is your insight on that?
A. As a chemist primarily focused on developing better fluorescent dyes, I don’t have a comprehensive vision of how AI will specifically transform cell biology and microscopy. However, I recognize that the field is undergoing rapid changes. I believe cell biologists, who are at the forefront of utilizing these tools, would be better equipped to articulate the evolving landscape and the potential impact of AI on their research.
While AI holds great promise, my personal focus remains on addressing the fundamental challenges in fluorescent dye chemistry that have persisted for over a century. These challenges include creating brighter, more specific, biocompatible, photostable, and less phototoxic dyes. Fluorescent markers are the cornerstone for high-quality images and videos, which are the core information for AI to infiltrate biological research. In this sense, we are building infrastructure for the AI era.
Q. Have you ever faced any specific challenges as an Asian researcher, while working abroad or anything related to doing science like publication? How do you think the cell biology domain can be more inclusive for Asian cell biologists and microscopists?
A. Working abroad as an expat, a minority, I did experience feelings of loneliness and isolation. This wasn’t intentional exclusion, but rather a natural consequence of being in a different cultural environment. Now, back in China, my home, those feelings have dissipated. I haven’t experienced any overt bias during publication related to my Asian heritage. However, it’s difficult to assess subtle biases without a controlled experiment. To foster inclusivity in cell biology and microscopy, I believe biology journals should actively involve more chemists and physicists, and vice versa. For example, chemists often need to publish in general chemistry journals for career advancement, even when their work has significant implications for cell biology. This creates a barrier to recognition and funding within the cell biology community. Breaking disciplinary barriers is crucial, even more so than overcoming scientific challenges. Together as a community, we should focus on this.
You can find all the posts from my interview series here.


 (No Ratings Yet)
(No Ratings Yet)