Technology highlights – Spinning Disk Microscopy
Posted by Johanna Bischof, on 14 October 2020
Interview with Stoyno Stoynov, Ph.D. from the Center of Advanced Microscopy, Bulgarian Academy of Sciences, Bulgaria.
Tell us a bit about the facility you run and what your focus is.
The Bulgarian Node of Euro-Bioimaging ERIC is based in the Institute of Molecular Biology of the Bulgarian Academy of Sciences, Sofia. Our node is part of and funded by the Bulgarian roadmap for scientific infrastructure. We specialize in live cell and functional imaging techniques to measure and model the kinetics of biochemical processes such as DNA repair and replication. In addition, our team has developed standalone image analysis software called CellTool which is ideally suited for the analysis of living cell datasets. CellTool combines not only multiple image analysis and tracking algorithms, but also offers a panel for mathematical modelling of the obtained experimental data. In this way, our node offers a complete set of open access services – from time lapse image acquisition to image analysis and mathematical modelling, for everyone who would like to study the mechanisms and kinetics of fast cellular processes.

We are today talking specifically about your application of Spinning Disk Microscopy (SDM) in studying DNA repair. Can you please tell us about how SDM works and what it can be used for?
Spinning-disk (Nipkow disk) confocal microscopes use hundreds of moving pinholes on a disc to simultaneously scan the specimen which ensures fast image acquisition and considerably reduces photobleaching and phototoxicity. This equipment is perfectly suitable to investigate almost every fast cellular process in living specimens such as cytoskeleton dynamics, vesicular transport, mitosis, etc. When the imaging capabilities of the spinning disk systems are combined with the ability to perform FRAP and UV laser micro-irradiation, a great deal of interesting results about the dynamics of cellular processes can be obtained in a short period of time.
Can you tell us a bit more about a specific project that was done in your facility using Spinning Disk Microscopy? What scientific questions were you addressing?
The main project that is being run in our facility for several years now is the investigation of the dynamics of DNA repair proteins at laser induced DNA damage foci in living cells. We recently reported the dynamics of 70 different DNA repair proteins in living cells that were recorded using a Spinning Disk Microscope following laser micro-irradiation induced DNA damage. That was the first comprehensive kinetics-based resource generated which offered unprecedented view of the timing of the DNA repair process and revealed some unanticipated events concerning the coordination between different DNA repair pathways in living cells. We are applying this approach to investigate the effects of various DNA repair targeting anticancer drugs onto the overall dynamics and efficiency of the repair process. This could lead to the discovery of novel mechanisms of action of these drugs and guide the development of better therapies for cancer patients.
We also have created an interactive online database – DNARepairK, containing all the obtained kinetic and image data which allows for easy comparison between the kinetics of recruitment of different proteins. You can read more about this work in this paper on Protein Dynamics in Complex DNA Lesions [1].
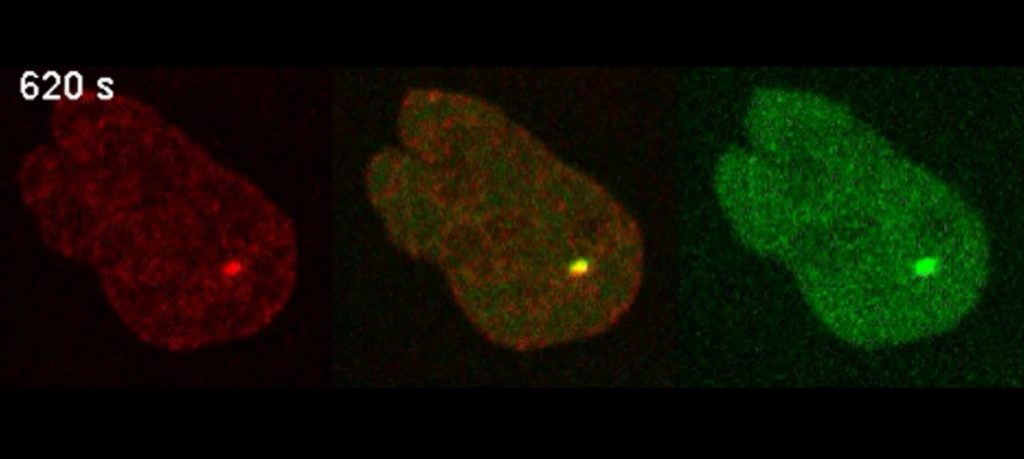
Why is spinning disc microscopy in combination with UV laser micro-irradiation best suited to address that question?
DNA repair is an amazingly complex cellular process with at least several hundred participating proteins organized in discrete DNA repair pathways. Our systems are equipped with UV laser micro-irradiation modules which allow for the generation of very precise DNA damage foci inside living cells and the observation of the recruitment of DNA repair proteins at those sites. Some of these proteins, e.g. the DNA damage sensor proteins, are recruited very fast to the damage sites and our equipment is capable of very fast imaging of these initial events following micro-irradiation so that we can obtain very precise kinetics even of such speedy processes. Other DNA repair proteins are recruited very slow, more than 15 minutes after damage induction, so we also need to perform some longer time-lapse experiments where we benefit from the extremely low photobleaching of our samples enabled. Overall, spinning disk confocal microscopy complemented with UV micro-irradiation is perfectly suitable for such kind of research since it combines high imaging speed with very low photobleaching and phototoxicity.
What are some challenges of this kind of microscopy? What do researchers have to pay attention to when performing these experiments?
There are several important things to consider. Probably the most important thing is that your cells must be feeling happy for good imaging – they must be at the right temperature, right growth medium, right humidity, right gaseous composition, etc. Even small deviations from the perfect growing conditions may lead to huge variations in the cellular process you are investigating. Our systems are equipped to provide the best possible growth conditions and we are used to running several-days-long experiments during which cells are dividing and growing optimally. Another important thing is the acquisition settings. Since we usually image cellular events that take place for several seconds to several hours or even days, we need to optimize the imaging settings so that we can obtain data with the best signal-to-noise ratio and with as many time points as possible, while in the same time diminishing the adverse effects that the excitation can exert on cells.
What other services do you provide in your facility that would be useful in combination with this type of microscopy?
If you are interested in the effect of certain cancer chemotherapeutics that target DNA repair processes, our facility is an expert in screening drug effects on the dynamics of the protein recruitment and interaction during DNA repair following UV laser micro-irradiation. For this, we have a wide range of cell lines that express fluorescent-tagged DNA repair proteins under their native regulatory sequences.
But UV laser micro-irradiation can be used not only for DNA damage induction in living cells, but also for cellular ablation and uncaging of specific substances inside cells. Our systems are equipped with modules for fluorescence recovery after photobleaching (FRAP) which is an amazingly useful technique in all of its variations to study the dynamics of proteins or other molecules in living cells. The facility also offers total internal reflection fluorescence (TIRF) microscopy which is suitable for observing fast events in the cell membrane or closely under it such as membrane lipids and/or proteins dynamics, cytoskeleton rearrangements, cellular junctions dynamics, etc., and widefield microscopy with deconvolution. And finally, from the superresolution techniques we offer SRRF-Stream super-resolution microscopy functionality employing low excitation energies with conventional fluorophores and D-STORM microscopy.
Want to use Spinning Disk Microscopy via the Euro-BioImaging service?
Euro-BioImaging is the European landmark research infrastructure for biological and biomedical imaging as recognised by the European Strategy Forum on Research Infrastructures (ESFRI). All scientists, regardless of their affiliation, area of expertise or field of activity can benefit from Euro-BioImaging’s pan-European open access services. By facilitating user access to high quality imaging facilities, resources, and services, with a constantly evolving technology offer, Euro-BioImaging will boost the productivity and impact of research across Europe.
Potential users of the Euro-BioImaging technologies are encouraged to submit project proposals via our website. To do so, you can LogIn to access our application platform, choose the technology you want to use and the facility you wish to visit, then submit your proposal. By using Euro-BioImaging, users will benefit from advice and guidance by technical experts working at the Nodes, training opportunities, and data management services.
For more information visit our website at www.eurobioimaging.eu or contact us at info@eurobioimaging.eu

Stoyno Stoynov
Center of Advanced Microscopy,
Bulgarian Academy of Sciences
Bulgaria


 (No Ratings Yet)
(No Ratings Yet)