An interview with Luis Felipe Barros
Posted by Mariana De Niz, on 22 November 2022

MiniBio: Dr. Felipe Barros is currently a group leader at the Center for Scientific Studies CECs and Professor at Universidad San Sebastián, in Valdivia, Chile, where him and his group study the flux of energy within cells in the context of metabolism, using and developing novel methods of fluorescence microscopy. Felipe obtained his MD and later his PhD in Biomedical Sciences at the University of Chile, where he worked in the lab of David Yudilevich. He then did a postdoc at the University of Leeds, UK with a Wellcome Trust Research Fellowship in the lab of Steve Baldwin. He returned to Chile in 1996 as an independent researcher, and has since received several accolades. He has been President of the Chilean Society of Cell Biology.
What inspired you to become a scientist?
This is a question that really made me think. But I see it a bit differently. Perhaps when we’re 8 years old, we are all scientists – we are inquisitive, we ask questions, we wonder about the world, we have no inhibitions when it comes to knowledge. Sadly, with time, many of us lose this innate curiosity, but some of us manage to recover it and become scientists. For me this was during University – where I studied Medicine. I realized many of my professors were rather unique. They had a different way of learning and a different approach to knowledge. I met one of them – Raul Domenech, who was a cardiologist. He told me he had his own lab, and invited me to see it. This was the first time I was in touch with a real scientist, not the textbook description of a scientist only. This was the first approach I had to science. By the end of my degree, I had to make a decision on what career to pursue (if Medicine per se, or science), but these teachers had left a mark. While studying Medicine I realized that being a physician and being a scientist are two very different things. The may look alike but at the core, they’re extremely different. And in the end the decision on what career to pursue was not easy. The life of a scientist and the life of a medical doctor diverge. Especially in low and middle income countries (LMICs), it’s difficult to lead these two lives. Both are very ‘jealous’ careers: they demand 100% of your time, energy and commitment. Some people have 200% to give, but I didn’t – I could barely manage one. So I ended up choosing science. At the end of my medical degree I got a fellowship, and I could choose whatever specialty I wanted. But I felt that I wasn’t ready. I loved Cardiology, but I wasn’t ready, and I thought to myself ‘I’ll do a MSc, to delay the decision’, and then the MSc came to an end, I enrolled into a PhD program and kept delaying the decision. At the end, it’s been 30 years and I still haven’t used the fellowship I earned to study Cardiology.
You have a career-long involvement in neuroscience, metabolism, and microscopy. Can you tell us a bit about what inspired you to choose these paths?
It was the people I met who inspired me to choose these paths. When I started my PhD, I had some extraordinary lecturers, who were working on membrane transport, particularly, Rosa Devés who later became a friend and a mentor for me, in the way of thinking and doing things. Because of her I gained interest in membrane transport. And there was something aesthetic about membrane transport that was very appealing. It has a mathematical component which I find fascinating. It has a stronger component of biophysics than other aspects of cell biology. Fiddling around with mathematical models, I realized I really enjoyed doing it. One thing led to another, and I ended up captivated by the field of membrane transport. I then did a postdoc in membrane transport too, investigating glucose and amino acids. At the time we were working with isotopes. Isotopes are great because they allow quantitation, but the resolution you can achieve is very limited. We could get a temporal resolution of 20 minutes, and a spatial resolution of 1 million cells. For you to get an idea, within the Department where I was working, there was a very good group working on electrophysiology – led by my other mentor, Francisco Sepúlveda. They could achieve extraordinary temporal and spatial resolution: basically information from the single cell level, in the millisecond scale. And it was here where my interest in microscopy began. I realized we were really far behind in the study of membrane transport, and other groups with different technologies could achieve much more detailed information. Unfortunately, some metabolites don’t have a charge, so you can’t measure them by electrophysiology techniques. So during my whole career, my inspiration has been to achieve better resolution – to obtain better quality measurements. As an independent researcher, I started developing methods to achieve this resolution in the field of membrane transport. Actually, developing methods has been the fuel behind the ideas that underlie scientific research. It’s been beneficial for us, because we can adapt microscopic techniques from other labs with a lot of ease, because we are always thinking about applications. I don’t think there’s a particular order on whether the method leads to the idea or the idea leads to the method, but I think they complement each other in a very harmonious way.
In terms of career chronology, I did my MSc at the Universidad de Chile, but I didn’t quite finish it because around this time the PhD program was created, and I was invited to join. So I transferred all my MSc work to the PhD thesis. My PhD was partly in the UK and partly in Chile. This was because of a transport expert who was coming back to Chile around that time, David Yudilevich – he wanted to start his lab at the Faculty of Medicine of Universidad de Chile, and he was looking for young students to start his lab. So I was lucky. During my PhD I got to travel to the UK. I must say when I was younger I really wanted to travel and get to see the world. I think the opportunity that science gives us to do this is fantastic and rather unique. I then met my wife, who is English, and so I pursued a postdoc in the UK – it seemed like the natural choice. I did a 3-year postdoc in Leeds with Steve Baldwin, mostly focusing on Biochemistry. By then I had a good idea of what the limitations of different tools were (of microscopy, of electrophysiology), and I knew that these tools were something important for me. But I was hesitant about my capacities as a molecular biologist. Yet it was very clear to me that molecular tools would become pivotal in the near future, and it was important for me to gain expertise in this area. I also wanted to gain expertise in protein biochemistry, but quickly realized I didn’t like this area. So I went back to physiology, already as a PI. I would define myself as a cell physiologist. In terms of Microscopy perhaps I’m a bit uncommon. I’m not so interested in the images per se, but rather in the information that photons carry about specific metabolites. I started with confocal microscopy. In my postdoc I had access to a confocal microscope that the University had just acquired and not many people used. I used to spend countless hours at the confocal, looking at structures simply out of fascination. Eventually I managed to combine microscopy with my interests in metabolism. These paths were somehow separate for a long time in my career. On one hand, I used to work with isotopes, which is what one could use at the time, and on the other hand, doing microscopy, using calcium probes or probes to investigate the mitochondrial membrane. I was able to look at live cells in real time. It was more of a hobby. Eventually, five or six years later, I realized I could combine my hobby with scientific questions, and something very interesting came out: tool development.
About my approach to microscopy: there’s a competition here in Chile, with the Society of Cell Biology, for the best microscopy image – I’ve never won it, nor have I ever competed. We image with as little intensity as possible so as not to damage the cell, and in order to obtain photons from specific cell compartments we are interested in. When we prepare papers, it’s not uncommon for one of the authors to say “shouldn’t we have a nice image of the signal of the sensor (eg. mitochondria and mitochondrial pyruvate)?”, but this suggestion often faces resistance. Nonetheless we do it, collaborating with our colleagues interested in structural biology. We are more interested in functional biology. But we are always happy to collaborate – we have even done intravital microscopy (IVM). For this, we have collaborators in Switzerland – the group of Bruno Webber in Zurich. We develop sensors, and Bruno and his lab do IVM, so it’s a two-way exchange of expertise. If we see something interesting, for instance, in neuronal function in vitro, the question arises on whether this happens in vivo too, and we work together to tackle the question. His group has a setup of 2PM with a robotic arm, which allows imaging animals in the awake state, and measure metabolites while the animal goes about its normal life. I find it fascinating that we can test how relevant in vitro observation are, when one moves to the in vivo setup. It’s fascinating biology that keeps us grounded. At the same time, a lot of the work we do tackles fundamental processes that at the time cannot be investigated in vivo because you can’t manipulate the setup: you can’t block certain enzymes or block transporters, so this will continue to be done in a reductive system. For the past year we have mostly used HEK cells, looking at mitochondria. Altogether, I do believe that different scales and different platforms help us answer different questions – it’s all complementary. It’s difficult for a single group to do all techniques and be an expert at all scales, so good collaborations are paramount. We’ve been lucky to have great collaborators whom we can trust. Trust is important in science, to be able to discuss freely and share data freely. Sometimes I have the impression that the scientific system is not designed to be collaborative, and this is a limitation we should be addressing. The incentives for fame and reputation and index factors, promote a culture of “my paper” vs “your paper”. I think this is all nonsense, and we should diminish this or vanquish it completely. Having a good collaborator is one of the best things one can find in science.
Can you tell us a bit about what you have found uniquely positive about becoming a researcher in Chile, from your education years?
I was lucky in that I had access to teachers who really cared about their students and who gave us a valuable ‘gift’: their time. It was a time when Chilean researchers didn’t yet have this ‘urge’ to publish 10 papers per year. My scientific career began after the dictatorship in Chile, and the feeling was more of a re-birth. Many researchers were coming back from Europe and the USA with a wish to contribute to Chile’s growth (or re-birth). They brought back the way of working from the First World, but would find here that the pace was slower. This slower pace allowed for more a more peaceful and more creative scientific process. This was the rule, rather than the exception, of the 1990s. There was time to philosophize, to discuss. Nowadays this is harder, because there is a ‘hunger’ for excessive productivity, and so I feel science becomes more prone to error and more superficial. At the time when I did my studies there were no worries about impact factors. No one expected you to publish in a big journal. Chile was very far from this. Today the expectations for young scientists are much higher, and more resources are used. People want more and more grants, and the personal factor in teaching and mentoring is lost. I see science as an art – it requires time. You cannot rush the artistic process. But I have seen science become an enterprise. However, while this is my opinion, I acknowledge that different people have different ways of doing things, and this is all respectable. But I feel the scientific world is more complicated now than it was back in 1990.
I have seen a transformation. We are always complaining that we have few resources, and it’s true, but resources can corrupt you- it’s not an easy trade. I was very lucky to be able to work in Valdivia (a city in southern Chile). The Centre where I work now, moved from Santiago to Valdivia in the year 2000, and we are a basic science Centre, with lots of time to do research. This gave me the luxury of being able to sit at the microscope for 6 hours every day. When I was doing my PhD, John Kendrew visited, and we had an interesting discussion with him (John Kendrew basically single handedly isolated the first protein for which a crystal structure was obtained- myoglobin). He told us he had worked for 15 years in trying to obtain the crystal structure of myoglobin, and until the day he got a crystal that diffracted, he didn’t know if those 15 years had been successfully used. He told us that in the 1990s, this type of science could no longer be done in First World countries, because most projects are funded for 3 to 5 years, and they require that you publish in high impact factor journals at the end of this time, so high-risk projects like the one he had done are very rare and not really encouraged. He said that in developing countries, the main advantage we have, is time. If you have one grant, you have a certain amount of time. But if you get 10 grants, you actually have a lot less time! You become a slave of your grants! It’s a good thing, in a way, that in Chile there’s a limit to the number of grants you can apply to. So you don’t end up becoming a slave of 10 grants. This is a blessing in disguise. Still, I would not mind if our grants were more substantial.
I think different countries have also a sort of dominant style: the USA for instance has a method that is not very efficient, but is very effective: you have 10 postdocs, and from those 10, 2 will have something publishable in any one year, so you have your papers. But for me this is an non-ecological type of science (and not good for most postdocs, who may become disillusioned). I think it’s great to be here and see the talent in our region.
Can you tell us a bit about your day-to-day work as a group leader at CECs?
I do different things every day. I’m very disorganized! The first thing I do is answer emails. Then I go downstairs to the lab to say hello to everyone, and receive questions and address anything that might be needed form me. At this point in my career, I’m more of a nuisance to my students. I do not longer do experiments with my own hands – so I go and bother them, ask them what they have done, what have they seen at the microscope and so on. A nuisance really! Regarding work that needs my concentration, I’m afraid that happens mostly at home on the weekends. If I need to write something, I do it at home. For example today, in Chile, it’s a holiday. So after this interview, I have to prepare a summary and two talks, and I love the fact that I have the full day to concentrate at home. If I’m at the lab, I get interrupted every 5 minutes, which is stimulating because I can discuss science with all my team. It’s just that it’s so hard to concentrate.
Something that my lab has been working towards is the generation of fluorescent sensors for metabolites. We have sensors for 5 o 6 metabolites, but the number of metabolites is in the order of thousands. So this is just starting. Now we can also see biochemistry happening in real time in a living cell, or in living tissue – no longer just in a test tube, we are getting rid of some pre-conceptions. I think this is essential in science. Every time we do an experiment with a new sensor we find something new– for example recently we used a sensor for citrate, and realized that there was something different about how mitochondrial citrate is regulated relative to how it’s supposed to be regulated. So this now will give us enough work for another 5 years. I imagine something similar happened with Astronomy and telescope designers. With each improvement, they would realize there were stars in places where they previously thought there was nothing. So they then would dedicate their lives to study the 3 new stars they discovered. So in microscopy, and its entry into biochemistry, I believe something similar is happening and will become stronger in the near future. And then microscopes last for a long time. Right now, in fact we are in a bit of a predicament. After 25 years with one of our microscopes, we just received notice from Olympus that the guarantee will soon be over and no more service can be provided. Basically the message is that if at any point this equipment fails, we’ll have to get a new one. But it’s been 25 years! We really take care of our equipment. For instance, someone who is careless with the microscope, becomes persona non-grata for a long time! Of course accidents do happen, but we are very careful and encourage this in the whole lab. Especially because most commercial microscopes are black boxes: you can’t really meddle with them so this can be a problem. I feel that the definition of what a microscopist is has evolved too. In the past, a microscopist was someone who could build microscopes. Now that’s considered a technical job, and the job of a microscopist involves many other things, but basically either you dedicate yourself fully to academic research, or you dedicate yourself fully to build microscopes. With very complex microscopes, it’s difficult to do both disciplines well. Even technicians are not experts in all microscopes – they specialize in one or two. Moreover, I feel nowadays if a microscope component is broken, many times, technicians from companies will offer to replace the part, rather than repair it. It’s the same in other things, like car tires for example: nowadays if a tire is damaged, you replace the tire. The job of repairing tires, that vulcanizers used to do, is no longer a big thing.

Did you have many opportunities to interact with other Latin American groups, outside of Chile?
Very few. We’ve put a lot of effort into doing this – we used to organize a course in the early 2000’s, to bring together Latin American students, precisely with the purpose of improving networking. But our countries here in Latin America are too big. The diversity of research topics is also huge, and so I feel it’s hard to find many groups that do the same – depending on the topic of course. But in my research field specifically, there aren’t even many labs doing similar things. I have collaborations with about 5 labs – most of them in Europe, and one in the USA. But more recently, we attend meetings, and we find more labs who are doing similar things. I’d love to encourage this networking in the region, but I honestly don’t know how. For instance, cells of great interest to my lab are glial cells – astrocytes – and someone tried to make a Glial Cell Society in Latin America, with the support of the International Glial cell community – we organized a meeting, but then this got dissolved. There’s little critical mass. Also science is no longer done in the same way it used to be in the past: in the past you chose one topic, and you focused on this topic your whole life. Nowadays it’s much more dynamic: you might belong to the Astrocyte community this year but next year your interest changes and now you are doing research in neurons. So what do you do? You no longer belong to the Astrocyte community 🙂 Anyway, every time I’ve tried to get something going it seems to lose momentum. It’s also not easy to travel. Distances in Latin America are huge: to go from Chile to Mexico, it’s 10 hours by plane. I once had a collaboration in Mexico, but it was less energized than I had hoped.
Who are your scientific role models (both Chilean and foreign)?
My role models are from old times. A historical character I find fascinating is William Harvey- he discovered blood circulation, and he is the father of Experimental Medicine. Something I find fascinating about what he did is that he was in the transition from Aristotelian Medicine, when you had to take dogmas that had been accepted for thousands of years, and challenge them. He was called ‘the ocular philosopher’, because he would make observations, and then would think, and then make observations again, and think. And this is something we do in my lab. He wrote a book I find amazing and I really recommend, in which he described his results about blood circulation- he described the whole process of changing ideas and challenging dogmas: he goes through the process of having some pre-conceived notion and transforming this. It’s not easy to fight against one’s own prejudices or preconceptions. In my lab we try to do this a lot.
More modern role models: for metabolism, my ‘spiritual leader’ is Otto Warburg – he was the father of modern Biochemistry 100 years ago. He had very clear ideas about what he wanted to do, and he pushed forward novel ideas that went against the tide. For this he was even ridiculed but at the end of the day he was right. And even more modern, Roger Tsien- we even have his picture in our lab. Tsien was very clear in that novel techniques needed to be developed (constantly) for science to advance. He proposed that one could be stuck thinking about concepts and hypotheses, but as long as you didn’t have the tools to answer your questions, you wouldn’t advance. In microscopy, as in any other area of modern science, it’s very hard to move forward without technological progress. It’s not enough to ‘think a lot’. I feel that in Cell Biology, we’ve barely just begun. It’s an ever-evolving discipline.
What is your opinion on gender balance in Chile, given current initiatives in the country to address this important issue. How has this impacted your career?
I don’t feel things have changed a lot over the past few years. When I receive undergraduate students in my lab, 50% of them are men and 50% are women. Once we reach PhD levels, there are already more men than women – something happens here and the ‘leaky pipeline’ starts. At postdoctoral level this worsens – it’s mostly men. When you start hiring junior faculty, there’s very few women. This is how it was when I was a student, and it’s how it is today. I don’t feel that at least in my area, things are improving fast enough. But I see oscillations within my own lab: in some past years, most of my lab members (at all levels) were men, and now they’re mostly women. I hope they carry on, but I see that something happens around the time they turn 30-35 years old, that they leave academia. It’s a pity because many of them are exceptional. I don’t understand why they are leaving. Regarding gender balance within the groups, I feel diversity is essential -it brings new ways of thinking, it bring better dynamics. But I don’t know how to improve retention of female talent.
Regarding childcare, this comes back to cultural expectations in Chile – where women are expected to be almost the sole caretakers. For instance, when the child gets sick, the woman is expected to take care of the child while the man is expected to go to work. While to cultural context does not change, women will continue to be affected by it: while the child is sick, the man continued to work 12 hours per day, went to all the meetings, and did all the traveling he needed to do. Meanwhile it was expected that the woman would not do any of those things while the child is sick. And this productivity difference is not even considered when she goes on to write a grant proposal. The context is toxic. I have friends who are women, who had to do enormous sacrifices for the sake of their careers, none of which myself or other friends (men) had to do. This is an insurmountable ‘delta’. Sadly also, what is happening is a reduction in births! Many couples choose not to have children, and this then tends more towards gender balance in terms of career progression.
What is your favourite type of microscopy and why?
My favourite is FRET. FRET is when you have a donor and an acceptor and you want to see if they interact. This is a complicated technique, but we do intramolecular FRET. We have the ligand, the protein that recognizes the ligand, a pair of FRET, and we express this on a cell – then we do FRET measurements, and we transform this into analyte concentrations. FRET sensors behave very well. They are ratiometric, which circumvents various possible experimental artifacts. There are some complexities, for instance if they are very big. Nonetheless FRET sensors are the standard in the field. Now we can target them to specific compartments, and see metabolites in the endoplasmic reticulum and mitochondria, in real time. This was unthinkable a few years back.. Also fluorescence lifetime imaging (FLIM) has been gaining momentum. But at the end of the day the limiting factor are our proteins, which are our sensors – and these sensors are not perfect. Thy change with the environment and with the pH. We are realizing what the limits are in the field, which are not really due to the microscopes, but rather, what we are observing: the sensors. But as I mentioned earlier, the area of sensors is also gaining momentum. At present there are thousands of metabolites which are invisible. Metabolomics does not have the spatial resolution that we want, because to do metabolomics you usually destroy the cell, and you no longer know what is coming from the ER or from the mitochondria, while microscopy allows for this spatial resolution.
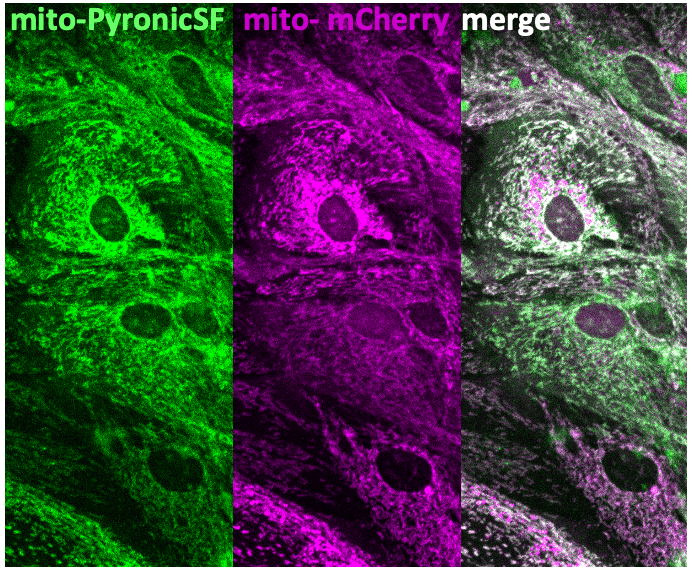
What is the most extraordinary thing you have seen by microscopy? An eureka moment for you?
It’s a difficult question. The way we do science in my lab is not that we’re looking for something specific. We allow ourselves to be surprised by unique observations, so I can’t really remember an eureka moment. It was rather a ‘wow’ moment – it’s a moment of surprise rather than confirmation and assurance. When we test hypotheses, there’s so much work involved that the happiness associated with the eureka moment is diluted and lost. It’s like we find the ‘e’ now, and then the ‘u’ three weeks later, and the ‘r’ two months later, and so on until the word is complete. By then the emotion is different. It’s never a single moment. We have to look for a different name for a ‘wow’ moment, which I think is different to eureka. I can think of Einstein with the anomaly of the Michelson-Morley experiment, where the speed of light was measured with the Earth moving in one direction or the other, and the speed did not change. Something like 2+2 equal 3.5. This was a ‘oh wait’ moment. Einstein took these results and thought, if these observations are correct, then we need to change the theory. This was a moment of surprise arising from an experimental observation.
What is an important piece of advice you would give to future Chilean scientists? and especially those specializing as microscopists?
Spend lots of hours at the microscope. Don’t try to save time at the microscope. Spend time observing and searching. Don’t be limited by the booking times of the microscope: look for times when it’s available. I feel sometimes some young scientists have little time- they’re too distracted, with the cell phone, with social networks. For instance, our typical experiments in the lab last about 2 hours. I remember when I was younger, there were no smartphones. So during the 2 hours of the experiment, I was dedicating my mind to divagate about the experiment. I feel millennials or newer generations want to use the time for something else: send an email, listen to music, send a tweet. I think a microscopist has to observe and has to think. But when I tell this to young researchers, this suggestion is met with derision. They think they can multitask, but for me multitasking is an unrealistic notion: it doesn’t exist. You can’t do two things at the same time with the highest quality. There might be geniuses out there who can, but the vast majority of the people can’t. I might be sounding now like a grumpy old man :), but this is how I see it.
Where do you see the future of science and microscopy heading over the next decade in Chile, and how do you hope to be part of this future?
I’m an optimist. Chile is going through an important political transition at the moment, and through an economic crisis too, as is the rest of the world. But I feel we’re behind in terms of science, and I feel it’s more likely we’ll be swimming upwards than sinking. I feel my task now is to help younger scientists to have the conditions and solid bases to do science in the near and long-term future. Perhaps the last 15 years I focused on myself and my science, but now I wish to be (and I feel I’ve become) a source of support for the younger researchers. Whatever I can help them with, with my experience, I am happy to do this. The language of science is English and therefore in Latin America, we have to work in a language that is not our own, and this has a negative impact. Moreover, I’ve focused on capacity building and infrastructure building so that the new generations have access to state-of-the-art technology for instance with the microscopes. It’s difficult because in our region resources are scarce. While the rate of improvement might be frustrating, I think we are all going forward on the slope. Maybe I’m naïve..Another problem I see is that many people within our region (in Latin America) don’t believe that we can do science with global relevance. This has impact on politics too. Moreover, in Latin America, the vision is many times short-sighted. It’s difficult for a politician to propose policies that will have tangible effects in 20 years, so they focus instead on things that will have immediate results. And we all know science is not immediate. But in this sense, for instance, we do have funding that allows for long-term research, with deliverables in a decade. This has been vital for many labs, including my own. I think this type of funding is important for the future.
Beyond science, what do you think makes Chile a special place to visit and go to as a scientist?
I live in a very special region of Chile, in the south, in Valdivia – at the beginning of the Patagonia. It has beautiful nature surrounding the city. We have sea, mountain, lakes, thermal waters, forest. If there are scientists who like nature, this is a beautiful country. In terms of culture, Chile is a country with growth pains – at present the history of Chile is not easy. I think many countries in Latin America are still finding their way, as opposed to say European countries where all the country’s machinery is already set and optimized. I think for young Latin Americans, this means they can still be part of shaping what their countries will become in the coming years. Valdivia has great food ! We have great seafood, great meat, great fruits and vegetables. It’s a fertile land. Valdivia is also a University town, so there’s always lots of cultural activities, music, theatre, etc. And the best beer of the country.


 (No Ratings Yet)
(No Ratings Yet)