How to Minimise Photodamage with LED illumination
Sponsored by CoolLED, on 15 November 2023
Both fixed and live samples are susceptible to photodamage during fluorescence microscopy, which can shorten time-lapse experiments and cast doubt on observations. LED illumination for widefield fluorescence has the potential to counter this serious problem, whether running a complete experiment or simply finding the region of interest prior to confocal microscopy.
Exposing biological samples to the excitation light levels typically used during a fluorescence microscopy experiment can have a detrimental effect, and countering photodamage is a crucial aspect of any fluorescence microscopy experiment.1 Photodamage comes in two forms:
Phototoxicity: Production of reactive oxygen species (ROS) during fluorescence excitation of live cells causes irreparable damage to biomolecules. The impact of phototoxicity can often be underestimated, since effects are not always immediately apparent. Cellular physiology can still be altered in subtle ways and can potentially lead to inaccurate conclusions, and the only way to truly detect phototoxicity is through experimental controls.
Photobleaching: An issue for both fixed and live samples, signal is gradually lost from fluorophores as some atoms absorb enough energy to break covalent bonds and irreversibly damage the chemical structure – whilst also releasing ROS. The speed of photobleaching varies between fluorophores, but it can result in both data misinterpretation and time lapse experiments being cut short, limiting the observation of longer processes.
How to counter photodamage?
A variety of strategies exist, from specialised media to selecting bright, stable fluorophores.2 Even when employing non-widefield techniques such as light sheet microscopy to minimise sample exposure, locating the region of interest often relies on widefield fluorescence. For the majority of light microscope experiments, it is therefore vital to understand how the widefield light source itself can be used to minimise phototoxicity and photobleaching.
- Less is More
The most obvious solution is to minimise the total light level at the sample plane. Although this applies to all light sources, traditional lamps can only be modulated by inserting neutral density filters, which makes it challenging or impossible to reach the minimum possible irradiance where the sample is just visible.
LED illumination systems instead allow fine irradiance adjustment using sliders either on a control pod, graphical user interface or imaging software (Figure 1). Irradiance can therefore be minimised to the exact level where structures of interest are visible. It is far better to start on the lowest irradiance setting and gradually increase this to the required level. Another useful feature of LEDs to look out for is individual channel control, limiting illumination only to the required wavelengths.
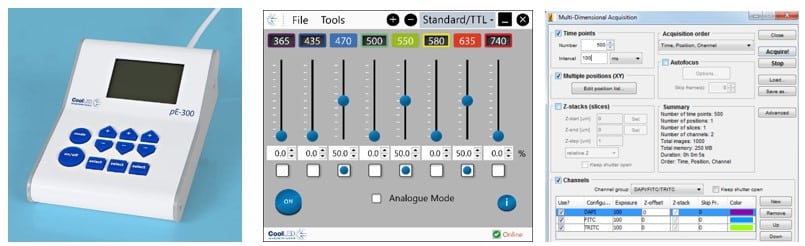
- Going Slow
For experiments where temporal resolution can be reduced, lowering irradiance and increasing exposure time is a widely applied principle that can minimise photodamage. It has been evidenced in several research studies (albeit using lamp illumination).3-6 Anecdotally, you can see in Figure 2 that it was also the most popular approach for minimising phototoxicity in an X (Twitter) survey from Dr Philippe Laissue, (65.2% answers of 112 votes).7

One proposed explanation is that by exposing the sample to a lower number of photons at any one time point, the cell’s detoxification systems can cope with clearing the harmful ROS. However, this is still a theory and experimental conditions can differ in their illumination sensitivities. In cases where temporal resolution can be reduced, the ease of trialling irradiance versus exposure time using an LED illumination system makes this approach worth investigating for fixed samples, and live cell imaging experiments which have controls to measure phototoxicity.8
- Reducing ‘Illumination Overhead’
Traditional lamps use mechanical shutters, and even when triggered electronically still create a lag which can be hundreds of milliseconds. A descriptive term for this is ‘illumination overhead’, and is defined by the authors as: “the time fluorescent samples are exposed to incident light, but fluorescence emission is not being collected by the detector”.6
Electronic LED triggering drastically reduces illumination overhead. Synchronising the illumination system and camera is possible using software control and achieves triggering speeds of up to 10 ms, depending on software and the PC operating system. Some LED illumination systems which feature a global TTL input can also be synchronised directly to cameras including a TTL-out, and this can be faster than 7 µs in the case of the CoolLED pE-800 Series. This approach is especially valuable over the course of time-lapse experiments and can result in improved cell viability and extended studies due to reduced photobleaching.
- Exploring the Spectrum
Traditional lamps tend to have a higher irradiance in the UV spectrum, which is more damaging towards samples, since these photons have higher energy and are more likely to be absorbed by biological materials. LED illumination systems offer far more choice in excitation wavelengths, for example 770 nm in the case of the CoolLED pE-4000 (Figure 3). These lower energy wavelengths are less damaging to live cells. Admittedly this approach is less simple, but as new fluorophores excited by longer wavelengths become available alongside the optical filters to match, re-assessing your LED, fluorophore and optical filter configuration may present another means of reducing sample photodamage.
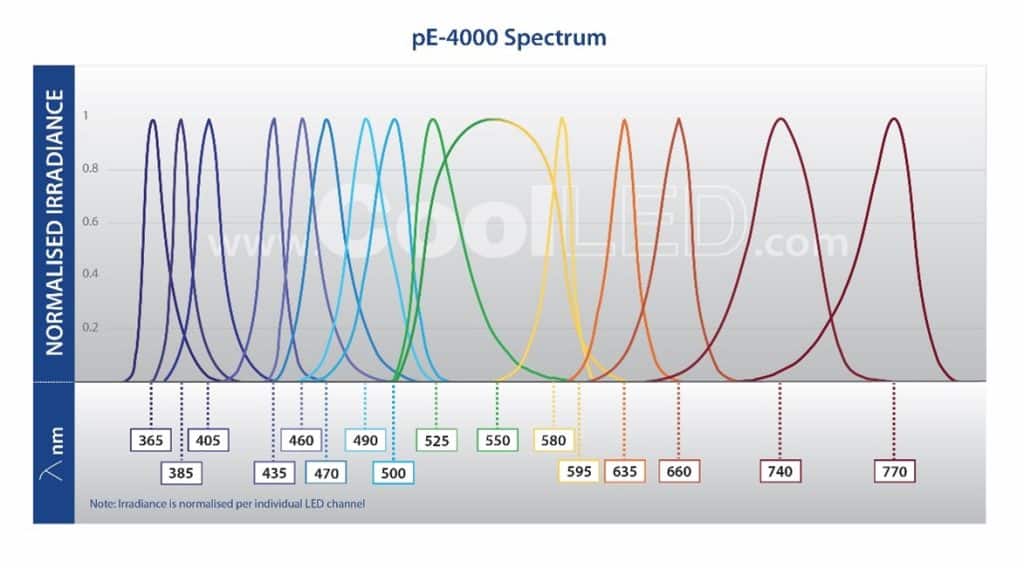
Conclusion
The wealth of research and increased awareness in the area of photodamage has highlighted the need for consideration in every imaging experiment, and the QUAREP-LiMi has a Phototoxicity working group to support this shift.9
Increasing numbers of labs and imaging facilities are upgrading from traditional lamps to LED illumination systems, making the features mentioned in this article available to scientists. Where it can be challenging to identify whether phototoxicity is impacting cell behaviour, it is important to err on the side of caution. Unfortunately, there is no ‘one size fits all’ approach and every experiment has different illumination sensitivities and requirements. For widefield fluorescence experiments, we therefore recommend taking the time to carefully optimise illumination settings or even investigate new fluorophore and optical filter combinations. Even when using widefield fluorescence briefly to set up confocal experiments, LED illumination systems can still help protect both fixed and live samples.
The topic of photodamage is broad and further advice can be found in many of the following references, and please do contact CoolLED if you have any questions regarding LED illumination.
References
1. Phototoxicity revisited. Nat Methods 15, 751 (2018). https://doi.org/10.1038/s41592-018-0170-4
2. Icha, J., Weber, M., Waters, J. C, & Norden, C. (2017). Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays, 39, doi: 10.1002/bies.201700003
3. Koester HJ, Baur D, Uhl R, Hell SW. 1999. Ca2þ fluorescence imaging with pico- and femtosecond two-photon excitation: signal and photodamage. Biophys J 77: 2226–36.
4. Tinevez JY, Dragavon J, Baba-Aissa L, Roux P, et al. 2012. A quantitative method for measuring phototoxicity of a live cell imaging microscope. Methods Enzymol 506: 291–309.
5. Dixit R, Cyr R. 2003. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J 36: 280–90.
6. Kiepas, A., et al. (2020). Optimizing live-cell fluorescence imaging conditions to minimize phototoxicity. Journal of cell science, 133(4), jcs242834. https://doi.org/10.1242/jcs.242834
7. Laissue, P. P. (2020). Phototoxicity poll. Twitter.
8. Laissue, P. P. et al. (2017). Assessing phototoxicity in live fluorescence imaging. Nature methods, 14(7), 657–661. https://doi.org/10.1038/nmeth.4344
9. Nelson G, et al., (2021). QUAREP-LiMi: A community-driven initiative to establish guidelines for quality assessment and reproducibility for instruments and images in light microscopy. J Microsc. 2021 Oct;284(1):56-73. doi: 10.1111/jmi.13041. Epub 2021 Aug 11. PMID: 34214188; PMCID: PMC10388377.



 (No Ratings Yet)
(No Ratings Yet)