When Microtubules Lose Their Grip: The Curious Case of CKAP5 and Chromosomal Chaos
Posted by Neelabh Datta, on 28 October 2024
Have you ever wondered what happens when cells lose control over their microtubule machinery during mitosis? This cellular machinery, when functioning properly, ensures that chromosomes are correctly segregated, preventing aneuploidy and mitotic catastrophe. However, when a protein called CKAP5, which plays a major role in maintaining the stability of microtubule attachments to kinetochores, is knocked out of the picture, things get rather chaotic. The depletion of CKAP5 causes microtubules to become unusually stable, leading to a cascade of errors in chromosome alignment and segregation. How, exactly, does the absence of this protein cause such widespread cellular havoc? A recent paper from Professor Tapas Manna’s lab at the Indian Institute of Science Education and Research, Thiruvananthapuram (IISER TVM), aimed to answer this question.
CKAP5 has long been known to be a key player in maintaining the proper dynamics of microtubules during mitosis. In human cells, when CKAP5 is reduced, microtubules attached to the kinetochores (KTs) of polar chromosomes become hyper-stabilized. Similar results were observed when cold-stable microtubules were analyzed in cells depleted of CKAP5. These microtubules, which are normally dynamic, become unusually rigid and stable, contributing to incorrect KT attachments. This over-stabilization has been shown to promote attachment errors between microtubules and KTs, with potentially devastating consequences for the cell. CKAP5, though known to help with microtubule polymerization, also plays a role in destabilizing the plus ends of microtubules. Without it, the cell’s microtubules can’t maintain the delicate balance needed for proper attachment, leading to attachment errors that can compromise chromosome congression and segregation.
One particularly intriguing consequence of CKAP5 depletion is the untimely recruitment of a phosphatase known as PP1 to the KTs. PP1 normally plays an important role in stabilizing KT–MT attachments and silencing the spindle assembly checkpoint (SAC) once the chromosomes have been properly aligned. However, when CKAP5 is depleted, PP1 arrives too early in the process, during prometaphase, and prematurely stabilizes erroneous attachments. This improper timing could explain why cells lacking CKAP5 often struggle with chromosome alignment and congression. Many chromosomes remain near the spindle midzone but fail to align correctly at the metaphase plate, likely because they are improperly attached. In such cases, the early arrival of PP1 may promote the stabilization of errors, such as merotelic or syntelic attachments, which were observed in CKAP5-depleted cells.
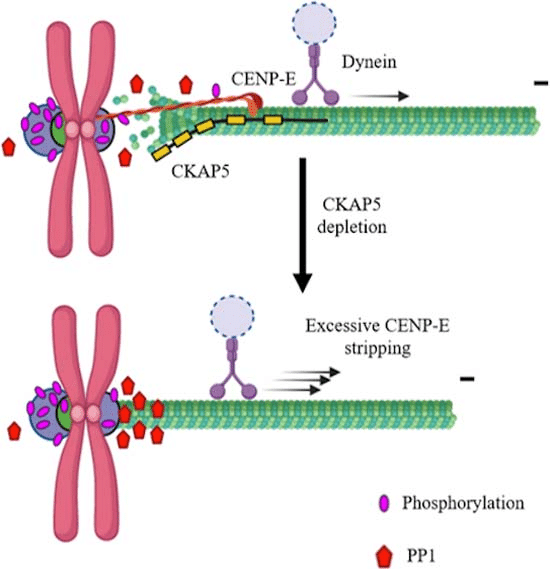
Depletion of CKAP5 leads to hyper-stabilized microtubules at kinetochores, increasing the recruitment of protein phosphatase 1 (PP1); this excess PP1 disrupts CENP-E localization, induces merotelic attachment errors, and destabilizes kinetochore function. Image adapted from: https://doi.org/10.1038/s44319-024-00106-9
How does the absence of CKAP5 promote the recruitment of PP1? It appears that CKAP5, through its regulation of microtubule stability, controls the timing of KT–MT stabilization and, in turn, the recruitment of PP1 to the KTs. Experiments have shown that expressing a truncated version of CKAP5, which contains key domains involved in microtubule interaction, can rescue both the excessive PP1 recruitment and the microtubule hyperstabilization caused by CKAP5 depletion. In other words, CKAP5 plays a direct role in managing microtubule stability and ensuring that PP1 arrives at the right place and at the right time.
Interestingly, CKAP5 depletion also appears to impact another KT-associated protein, CENP-E, which is essential for chromosome congression. In normal cells, CENP-E helps guide chromosomes to the metaphase plate, ensuring that they are properly attached to both spindle poles. However, in CKAP5-depleted cells, CENP-E is prematurely removed from KTs, likely due to the mismanagement of microtubule stability and the untimely recruitment of PP1. Studies have shown that drugs like paclitaxel, which stabilize microtubules, do not cause CENP-E removal in control cells, further highlighting the unique impact of CKAP5 depletion on this process. It is possible, that the hyperstabilized microtubules induced by CKAP5 depletion create conditions that favor the stripping of CENP-E from the KTs, either through increased dynein-mediated movement or other mechanisms.
Further experiments demonstrated that a brief treatment with nocodazole, a drug that disrupts microtubules, could partially rescue the loss of CENP-E from KTs in CKAP5-depleted cells. This suggests that CKAP5 is crucial for maintaining an optimal balance between microtubule stability and the recruitment of proteins like PP1 and CENP-E to the KTs. A mutant form of CKAP5 that causes hyperstabilization of microtubules also leads to significant loss of CENP-E from partially aligned KTs, further supporting the connection between microtubule stability and CENP-E retention.
The relationship between CKAP5, microtubule stability, and CENP-E becomes even more interesting when considering the role of BubR1, a protein that interacts with and stabilizes CENP-E at the KTs. In CKAP5-depleted cells, this interaction is disrupted, resulting in the removal of CENP-E from the KTs. However, when CKAP5 or PP1 is restored to normal levels, the interaction between CENP-E and BubR1 is also rescued, highlighting the critical role that CKAP5 plays in maintaining these interactions.
The loss of CKAP5 has wider implications for the cell’s ability to correct errors during mitosis. The improper stabilization of microtubule–KT attachments in CKAP5-depleted cells leads to an increased frequency of syntelic attachments, where both sister chromatids are attached to the same spindle pole. These syntelic chromosomes are unable to congress to the metaphase plate, instead remaining near the poles, unable to experience the necessary pulling forces from opposing spindle poles. The scattered distribution of syntelic chromosomes observed in CKAP5-depleted cells is consistent with the hypothesis that improperly stabilized KT–MT attachments prevent proper alignment and bi-orientation.
Computational models further suggest that the weakening of the forces between microtubules and KTs, caused by the loss of CKAP5, leads to an increased likelihood of error-prone attachments. These models predict that around one-third of chromosomes in CKAP5-depleted cells will exhibit syntelic attachments, consistent with experimental data. Moreover, the thicker microtubule bundles near the metaphase plate in CKAP5-depleted cells could result from slower turnover of KT–MT bonds, leading to an accumulation of attachments.
In the context of cancer biology, CKAP5 depletion has been found to be particularly detrimental to chromosomally unstable cells. Chromosomally unstable cancer cells are more sensitive to CKAP5 depletion, which exacerbates their mitotic errors. Interestingly, CKAP5 depletion was also found to correlate with sensitivity to CENP-E depletion, further highlighting the functional relationship between these two proteins. The findings suggest that CKAP5 might serve as a synthetic lethality target in aneuploid cancer cells, offering potential therapeutic opportunities.
This raises a final question: Could targeting CKAP5, a seemingly humble microtubule-associated protein, provide a new way to exploit the vulnerabilities of chromosomally unstable cancer cells and push them over the edge into mitotic catastrophe? One can only hope. After all, who would have thought that a single protein could have such a monumental impact on the fate of dividing cells?
Original Paper
Lakshmi, R. B., Nayak, P., Raz, L., Sarkar, A., Saroha, A., Kumari, P., Nair, V. M., Kombarakkaran, D. P., Sajana, S., M G, S., Agasti, S. S., Paul, R., Ben-David, U., & Manna, T. K. (2024). CKAP5 stabilizes CENP-E at kinetochores by regulating microtubule-chromosome attachments. EMBO reports, 25(4), 1909–1935. https://doi.org/10.1038/s44319-024-00106-9


 (No Ratings Yet)
(No Ratings Yet)