“openFrame” for modular, extensible, easily maintained, open-source microscopy
Posted by Sunil Kumar, on 23 July 2020
The openFrame [1] is an open-source microscopy hardware project initiated by the Photonics Group in the Physics Department at Imperial College London that is intended to provide access to a modular, upgradable, easily maintained and modifiable microscope frame that can be implemented at relatively low cost compared to existing commercial instruments. openFrame is a component of our “openScopes” programme [2] to make advanced microscopy techniques more accessible and sustainable, including in less affluent countries where maintaining commercial instrumentation can be even more challenging than the initial purchase.
openScopes was originally conceived as a way of sharing our implementations of automated (multiwell plate) microscopy, fluorescence lifetime imaging microscopy (FLIM), optical projection tomography (OPT) and single molecule localisation microscopy – for which we introduced a low-cost implementation of dSTORM that we call “easySTORM” [3]. Implementing these microscopy techniques typically entails assembling different modular hardware components, such as excitation sources, launchers, cameras and electronics, combined with open source software to control the instruments (e.g. Micro-Manager [4]) and to analyse the acquired data (e.g. ImageJ [5] and associated plug-ins). Some of the hardware components can be self-built and others are commercially available. We hope that openScopes can help the wider community to share ideas, know-how and components for the implementation and application of a range of microscopy techniques. Research groups can combine purchased and self-made components in their instruments according to their specific requirements and local capabilities.
Our design to implement easySTORM as an upgrade to a commercial microscope was originally implemented using inverted fluorescence microscope frames from Olympus, and the modular approach can be extended to microscope frames from other manufacturers and/or to other modalities, e.g. using add-on modules for excitation lasers, illuminators and camera systems, etc. However, adapting to, and working around, the commercial microscope frame can present challenges and sometimes compromises functionality. This led us to develop an open microscope frame that can be rapidly fabricated using standard machining techniques and which is easily extendable and modifiable to enable a range of imaging modalities. The openFrame also makes it relatively cheap to build a microscope from the ground up and the relatively simple design confers superior stability to previous home-built microscopes in our experience. It is compact, enabling complete microscopes to be implemented in standard cell incubators or high-level containment facilities. As well as benefitting lower-resourced research communities, we believe that the openFrame concept will be of interest to leading microscopy laboratories, since it allows rapid testing and prototyping of novel microscope configurations, and it could also be of interest to industry, e.g. SMEs developing commercial microscope components that can be implemented with openFrame to realise cost-effective bespoke microscope systems. We also hope that openFrame can lead to lower-cost and more easily maintained instruments for clinical applications such as histopathology. We aim to provide guidance for researchers wishing to implement a variety of imaging techniques using an openFrame. These will include component lists and links to download the relevant open source software.
The openFrame concept is based around a layered cylindrical geometry. This design provides modularity and flexibility, with each layer providing specific functionality, and layers can be stacked as required to implement a particular microscope configuration. The layers have a standard diameter of 150 mm and can be readily fabricated using CNC milling machines, but fabrication using standard lathes and milling machines should also be possible. The minimum mass of a 3-layer microscope is 10 kg, which confers a high degree of stability that compares favourably with commercial microscope frames (as measured by monitoring drift of beads on a sample stage over a 5-minute time-course image acquisition). Each openFrame layer includes face plates to which optical cages, tubes, or other components can be mounted, enabling coupling to cameras, optical fibre couplers, or other optical sub-systems.
Exploiting the circular geometry, the layers can be precisely co-aligned to be centred on a vertical optical axis, which can be readily defined using apertures, providing a convenient reference for aligning optical components. The system is designed to use a range of widely available (and reusable) filter cubes that can be rapidly changed, and the optical system can be configured for telecentric epi-illumination and imaging.
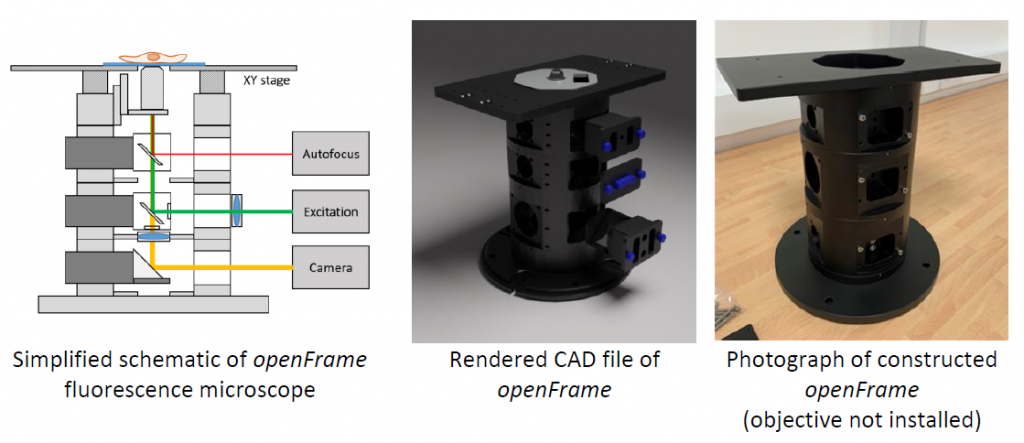
Functionality can be extended by introducing new layers. For example, additional imaging layers can be added to the infinity space for multispectral imaging, and further capabilities could be implemented by designing and fabricating new openFrame layers. The current set of available openFrame layers are sufficient to enable the assembly of conventional inverted fluorescence microscopes. We have shared designs for their extension to easySTORM on our website and will soon share designs for automated (multiwell plate) imaging – for which an optical autofocus module is in development. We are openly sharing the designs for the basic openFrame and also expect future more sophisticated layers, e.g. with motorisation, to be developed as products by commercial manufacturers. The openFrame should also be compatible with a wide range of open hardware already available or in development, e.g. for liquid handling, automated feed of immersion fluid, environmental control, etc. We are also developing a laser interlock system to help users ensure safety, e.g. when using high power lasers for single molecule localisation microscopy.
We currently control microscopes built around openFrame using Micro-Manager, as is the case for most of the devices within the openScopes portfolio. Micro-Manager is an open source software platform for microscope control that provides a convenient user-friendly graphical user interface for a variety of imaging modalities. Where possible we use existing Micro-Manager plug-ins, such as the “multidimensional acquisition” plug-in for fluorescence microscopy and easySTORM, and we are writing new plug-ins for modalities such as multiwell plate imaging, wide-field time-gated FLIM and OPT. These will interface with functionality already included in Micro-Manager and will extend some image modalities, e.g. to automated imaging for High Content Analysis (HCA). Our image data analysis typically utilises ImageJ, for which we have written plug-ins, e.g. for parallelised processing of STORM data, or MATLAB, which is used for FLIMfit [6], our open source FLIM data analysis software.
The current openFrame was designed in a collaboration between the Photonics Group and Optomechanical Instrumentation Workshop in the Physics Department at Imperial College London and Cairn Research Ltd [7]. The computer-aided design (CAD) files for the first openFrame design were made by Simon Johnson at Imperial, where Frederik Görlitz implemented and tested the first prototype. The CAD files for the second (current) design were made by Frederik Görlitz and Callum Hollick at Cairn, with input and feedback from Sunil Kumar (who constructed and tested the prototype at Imperial) and Paul French (Imperial) and Jeremy Graham (Cairn). Chris Dunsby, Martin Kehoe, Mark Neil and Jonathan Lightley from Imperial also contributed to the designs.
The openFrame CAD files are available for download [1] for anyone who wishes to make their own openFrame components, or alternatively, individual layers or entire systems can be purchased from Cairn Research Ltd.

Sunil Kumar
Postdoc at Imperial College London
Photonics Satellite Lab
The Francis Crick Institute


 (No Ratings Yet)
(No Ratings Yet)
