Technology highlights – Coherent Anti-Stokes Raman Spectroscopy (CARS)
Posted by Johanna Bischof, on 28 October 2020
Interview with Antti Isomäki, PhD from the Biomedicum Imaging Unit of the University of Helsinki, Finland and Dalibor Pánek, PhD from the BIOCEV in Prague, Czech Republic.
Tell us a bit about who you are and where your facility is based.

AI: My name is Antti Isomäki and I am an optical physicist by training. The path to my current position went through basic research in a laser lab, postdoc time in microscopy technology development, and finally biomedical applications and imaging method development. I have been working at the Biomedicum Imaging Unit for some eight years now and, as my main role, I provide support for the label-free nonlinear imaging techniques. Our core facility is located at the medical campus of the University of Helsinki, part of the Euro-BioImaging Finnish Advanced Light Microscopy Node.

DP: My name is Dalibor Pánek. I obtained my Ph.D. in applied physics. A year ago I joined the Imaging Methods Core Facility at BIOCEV, a member of the Euro-BioImaging Prague node, as a specialist in optical microscopy. I provide training and support for conventional techniques (confocal, TIRF, wide-field epifluorescence) as well as advanced fluorescence microscopy methods (FLIM, multi-photon, imaging FCS, FRET).
We are today talking about Coherent anti-stokes Raman spectroscopy (CARS). Can you please briefly explain how CARS works and what some possible applications are?
DP: CARS microscopy utilizes nonlinear interactions of two laser beams to image structures based on their vibrational properties, i.e., the contrast is provided by chemical composition of the observed structure. CARS is a point scanning technique and does not require fluorescence labeling or any special treatment of the sample and can be used on live samples. The resolution of CARS microscopy is about the same as for 2-photon microscopy, so sub-cellular structures can be imaged.
In the case of our in-house-built set-up at BIOCEV in Prague, we are somewhat limited by technical specifications of our equipment to detect CARS signal of vibrations of -CH2 (around 2870 cm-1). This allows us to image structures with high lipid content.
AI: CARS microscopy indeed offers complimentary contrast in samples where we do not want or cannot use fluorescent labels. As the technique is based on resonantly driven intrinsic molecular vibrations, the strength of the observed signal depends heavily on the molecule concentration in the focus.
In the beginning, our main applications of CARS at the Biomedicum Imaging Unit in Helsinki were related to imaging lipid droplets in fixed and live cells. Another promising application has been imaging unstained tissue sections, taken from e.g. liver biopsies, where CARS signal from lipids and second harmonic generation from collagen fibers can be detected simultaneously.
More recently, CARS has proved to be extremely powerful in imaging pharmaceutical materials. We have demonstrated high-resolution chemically specific imaging of drug microparticles in cell cultures and tissue. In pharmaceutical material development, CARS can distinguish between various non-fluorescent materials, or even between the polymorphs of the same drug material. This information is very useful when, for example, tablets with optimal dissolution profiles are developed.
Tell us a bit more about a specific project that was done in your respective facilities using CARS? What scientific questions were you addressing?
AI: Researchers from the field of pharmaceutical chemistry and technology have combined CARS with various other methods to analyze drug crystallization and its influence in dissolution of tablets. Crystallization during storage and administration is a major problem that affects the efficacy of the drug. Using CARS it was possible to detect subtle amounts of crystals, which may be important when developing new drugs and dosage forms [1].
DP: The CARS system in our facility is used mainly by the Molecular pathology research group lead by Z. Kostrouch from the 1st Medical Faculty of the Charles University in Prague. In their previous work, they used CARS to visualize lipid structures in C. elegans to study the effect of a particular nematode protein on lipid metabolism, as illustrated in this publication on Perilipin-related protein regulates lipid metabolism in C. elegans [2]. They continue this research using the CARS set-up in our facility. However, our data were not published yet.
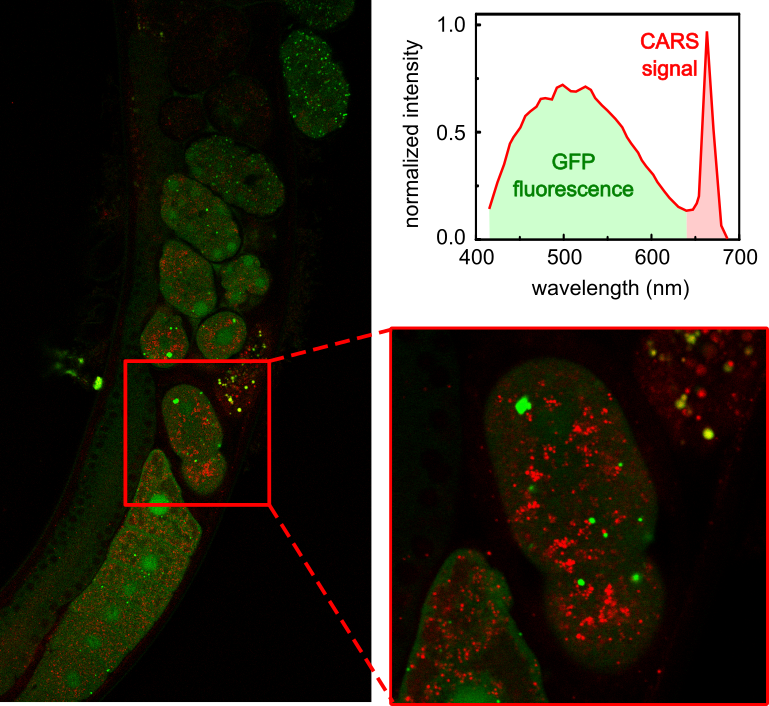
Why is CARS best suited to address these kinds of questions?
DP: Our applications focus on visualizing lipid structures, because the specific fluorescent labeling of lipid droplets is problematic. Labeling itself can affect the structure and function. Therefore, lipid visualization by fluorescence microscopy can yield inconclusive results. CARS is label-free while it provides a reliable way to image lipid-containing structures. Of course, combining CARS microscopy with other complementary imaging techniques is the most advisable approach.
AI: In our case, where we looked at drug crystallization, CARS together with other nonlinear microscopy modalities allowed for fast, high-resolution imaging of very early stage crystallization that was not detectable with the conventional solid-state analysis methods such as XRD or ATR-FTIR.
What are some challenges when using CARS? What do researchers have to pay attention to when performing these experiments?
AI: Often the sample preparation is easier than with fluorescence-based methods – you simply have your unstained cell culture, tissue or other sample on a cover slip. Image scanning works largely the same as in confocal microscopy. However, the interpretation of the resulting images requires some careful consideration due to the nonlinear nature of the method. For example, quantitative measurements are challenging. CARS is also not as sensitive as fluorescence imaging, so one should not expect single molecule precision.
DP: CARS microscopy is a laser scanning technique. Therefore, the user must be prepared to wait a bit to acquire a nice image. This also implies that the living sample must be immobile/stationary. The CARS set-up is also rather complex. The alignment procedure as well as operation are not straight-forward. The researcher usually needs our assistance with the measurement.
The researcher should also be aware that CARS is a four-wave mixing process and as such it is a bit tricky. The intensity of CARS signal depends on the distance of the focal plane from the coverslip so the 3D reconstruction from a z-stack cannot be performed simply as one is used to from confocal fluorescence microscopy.
What other services do you provide in your respective facilities that would be useful in combination with this type of microscopy?
AI: The imaging facilities in Euro-BioImaging’s Helsinki Node provide access to most of the commonly used microscopy techniques varying from in vivo optical microscopy to electron microscopy. Therefore, multimodal imaging combining CARS with other techniques is possible here. We collaborate also with other facilities and departments and can help getting access to additional sample preparation and analysis methods.
DP: We, at the BIOCEV in Prague, implemented CARS modality to extend further the capabilities of already quite powerful laser scanning microscope with a choice of spectral and time-resolved detection. Therefore, multiple techniques can be readily combined in a single multi-modal set-up: conventional fluorescence confocal imaging, 2-photon-excited fluorescence, second-harmonic generation, and 2-photon-excited fluorescence lifetime imaging (FLIM).
Why should scientists choose your facility for using this technology?
DP: CARS microscopy is still a sort of specialty, commercial systems are quite expensive and as such it is not widely available, and even more rarely offered as an open-access service. A special motivation to use our facility for CARS is the option of the multi-modal acquisitions. And of course, scientists can plan their complex experiments not only for a CARS approach, but can consider other methods in the rich portfolio offered by our facility.
AI: We were among the first sites to start working with the commercial CARS microscope from Leica. During the years, we have tested quite a large number of different samples and got a good understanding of the possibilities and limitations of the technique. I am always ready to discuss the feasibility or run a pilot test to see if our instrument works for certain application. Finally, I should mention that some new instrumentation for CARS and stimulated Raman spectroscopy (SRS) will be available in Helsinki next year. I hope that by that time the scientists will be able to travel here more freely again.
Want to use Coherent Anti-Stokes Raman Spectroscopy (CARS) via the Euro-BioImaging service?
Euro-BioImaging is the European landmark research infrastructure for biological and biomedical imaging as recognised by the European Strategy Forum on Research Infrastructures (ESFRI). All scientists, regardless of their affiliation, area of expertise or field of activity can benefit from Euro-BioImaging’s pan-European open access services. By facilitating user access to high quality imaging facilities, resources, and services, with a constantly evolving technology offer, Euro-BioImaging will boost the productivity and impact of research across Europe.
Potential users of the Euro-BioImaging technologies are encouraged to submit project proposals via our website. To do so, you can LogIn to access our application platform, choose the technology you want to use and the facility you wish to visit, then submit your proposal. By using Euro-BioImaging, users will benefit from advice and guidance by technical experts working at the Nodes, training opportunities, and data management services.
For more information visit our website at www.eurobioimaging.eu or contact us at info@eurobioimaging.eu



Dr. Dalibor Pánek
Imaging Methods Core Facility
BIOCEV
Prague


 (No Ratings Yet)
(No Ratings Yet)