Technology highlights – Traction Force Microscopy (TFM)
Posted by Johanna Bischof, on 9 December 2020
Interview with Aki Stubb, Ph.D.
Please tell us a bit about yourself and the facility where you work.

My name is Aki Stubb and I am a post-doctoral fellow at the University of Cambridge, UK. I did my PhD in the group of Johanna Ivaska at the Turku Bioscience Centre, University of Turku and Åbo Akademi University in Turku, Finland.
The work we are discussing today was done during my PhD studies there, in collaboration with the Turku BioImaging Center which is part of the Finnish Node of Euro-BioImaging.
We are today talking about super-resolution Traction Force Microscopy (TFM). Can you please briefly describe the principle behind TFM and how it can be used?
Traction force microscopy (TFM) is a well-established technique for measuring forces exerted by the cells on their surrounding extra cellular matrix. In TFM the living cells are seeded on elastic gels (usually polyacryl amide) with known biophysical properties, such as elasticity and stiffness. In the casting stage fluorescent beads are embedded into the gels. When the cells adhere to the gels they displace the gels alongside with the embedded beads. The movement of fluorescent beads can then be tracked using confocal or widefield microscopy and any number of time points can be acquired (“pre” images). The cells are finally detached from the gel and reference picture is taken (“post” image). The beads are then computationally tracked from the images and displacement is obtained by comparing to the reference picture. The traction forces exerted by the cells on the matrix can then be calculated from the beads displacements using mathematical frameworks such as Fourier transform traction cytometry (FTTC).
In a recent project you specifically developed a super-resolution approach to TFM. What is the difference between super-resolution TFM and normal TFM?
In super resolution TFM the fluorescent beads used are smaller and they are only cast in the thin top layer of the gels. In addition, the acquisition of the bead images is done using a super resolution imaging modality called super-resolution radial fluctuations (SRRF) which utilises the intrinsic radial fluctuation of the fluorophores to precisely determine the location. By using this method for imaging, larger number of traceable beads can be recognised and used for the calculations. This enhances the spatial resolution of the force measurements.
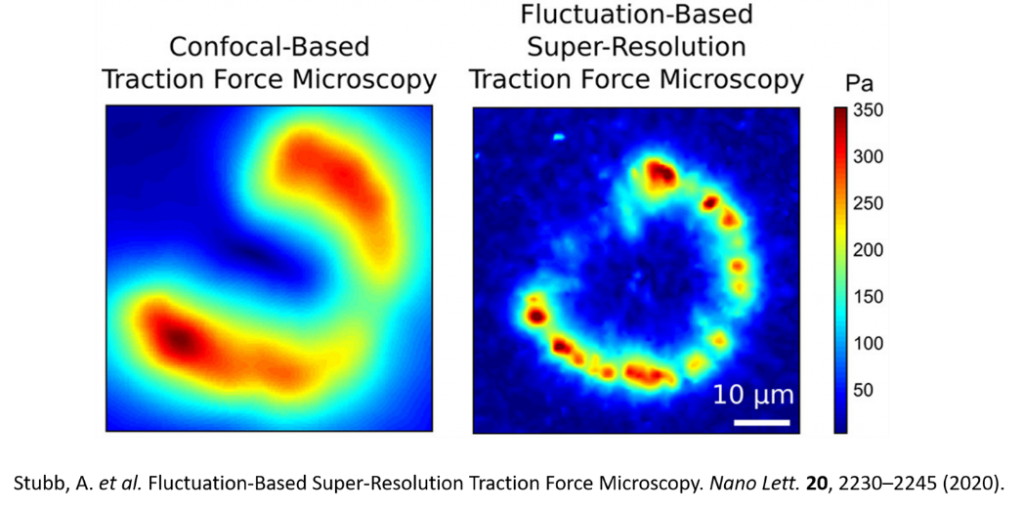
The method can be used for live cells measurement since SRRF is a gentle SR modality. As the method also improves the spatial resolution of the measurement it can be used to measure forces from individual subcellular structures such as focal adhesions. You can see the difference in resolution in the Figure below, taken from the paper where we describe the method [1].
In addition the technique can be applied to measurement of tractional forces from larger field of view (400um x 400 um) using 20x objective and widefield microscope.
Can you briefly explain the principle behind the super-resolution method, which is based on fluorophore fluctuation, that you used in this study?
SRRF or super resolution radial fluctuations is a relatively new super resolution modality, and it relies heavily on the computational processing of the images. Usually 100 frames of a given area of interest are taken in a rapid sequence and the radial fluctuations are determined using either confocal or widefield microscopy equipped with scientific grade camera. EMCCD camera seems to work best. Subsequently, the images are processed to calculates the degree of local gradient convergence (radiality) and in this way improve the resolution. This analysis is done in Fiji and run through GPU powered SRRF plugin. The plugin generates the super-resolved image that is then used the TFM analysis.
What scientific questions are you addressing using super-resolution TFM in your research?
This method was originally generated for improving the spatial resolution of TFM for measuring the forces from individual focal adhesions in photosensitive cells such as human pluripotent stem cells (hPSCs).
What are some challenges of using TFM generally and in combination with super-resolution microscopy? What do researchers have to pay attention to when performing these experiments?
The preparation of the gels is usually the biggest hurdle and, for this method, specifically it is important to get the highest-quality gels. The stiffness of the gels needs to be validated using atomic force microscopy. Furthermore, it needs to be made sure that the fluorescent beads at the top layer of the gels is uniformly distributed. In addition, the imaging and SRRF processing of the samples needs to be optimised.
What other services do you provide in your facility that would be useful in combination with this type of microscopy?
Super-resolution TFM requires a combination of microscopy methods that are all offered at the Finnish Advanced Light Microscopy Node of Euro-BioImaging with whom we have been collaborating for this project. The facility offers fast and high-quality confocal microscopy and/or spinning disk microscopy methods needed to image the fluorescent beads embedded in the hydrogels, same as the atomic force microscopy methods needed to calibrate the stiffness of the gels, and the actual TFM mathematical analysis needed for the final results. Furthermore, the facility offers full support and personalised service on all the steps, from gel preparation to data analysis.
Want to use super-resolution Traction Force Microscopy via the Euro-BioImaging service?
Euro-BioImaging is the European landmark research infrastructure for biological and biomedical imaging as recognised by the European Strategy Forum on Research Infrastructures (ESFRI). All scientists, regardless of their affiliation, area of expertise or field of activity can benefit from Euro-BioImaging’s pan-European open access services. By facilitating user access to high quality imaging facilities, resources, and services, with a constantly evolving technology offer, Euro-BioImaging will boost the productivity and impact of research across Europe.
To be able to offer its users the newest technologies, Euro-BioImaging constantly assesses new imaging technology developments in a process called showcasing. Super-resolution Traction Force Microscopy is currently under showcasing and available for users to test at the Finnish Advanced Light Microscopy Node. If you are interested in using this technology in your research, please contact the Node directly. As usual, users will benefit from advice and guidance by technical experts working at the Nodes, training opportunities, and data management services – and the user experience contributes to the inclusion of these new technologies in Euro-BioImaging’s operational portfolio.
For more information visit our website at www.eurobioimaging.eu or contact us at info@eurobioimaging.eu


Aki Stubbs
University of Cambridge
Cambridge, UK


 (No Ratings Yet)
(No Ratings Yet)