Phototoxicity – the good, the bad and the quantified.
Posted by Philippe Laissue, on 14 May 2021
Our virtual meeting on phototoxicity was held in late January 2021, generously sponsored by the European Microscopy Society and enabled by the Royal Microscopical Society. In four hours, spread over two days, the five organisers and twenty invited participants discussed the problem of phototoxicity in live imaging, and how we can start to tackle this as a community*. This blog article shares the major conclusions we collectively drew from this very insightful, enjoyable and fruitful meeting. Please note that this is a meeting report and not a review – articles on how to assess, minimise and report phototoxicity are referenced here for the interested reader [1-5].
*The discussion was partly based on a Twitter poll series we ran previously, the results of which can be found here. Many thanks to everyone who participated in the polls!
In the context of live fluorescence microscopy (rather than dermatology), phototoxicity describes the phenomenon by which the light used for fluorescence excitation leads to physiological changes in the observed living sample, be that single cells from a cell culture or a multicellular organism such as a zebrafish. With the excitation light intensities widely used in fluorescence microscopy, these physiological changes are often severe and detrimental and may lead to significant alterations in the biochemistry, physiology and dynamic behaviour of the observed sample. It is also possible that, when observing physiological processes for the first time, more subtle phototoxic effects may go unnoticed as the unperturbed activity is unknown. In either case, the conclusions drawn from these observations could be erroneous and, more dangerously, misleading – since we are not observing a living sample in homeostasis, but documenting the light-induced pathophysiological changes caused by the microscopy method.
We invited 20 participants from a broad range of research fields, some of whom gave short talks on their areas of expertise ranging from instrumentation and deep learning to biological readouts of phototoxicity. The speakers were also asked to share what in their view needed to be addressed most urgently, and if they could already think of solutions to these challenges. These thoughts and their talks were the basis for the discussions that followed.
So here are a few points we came up with in the meeting:
Raise awareness. Phototoxicity is not widely addressed in scientific publications using live imaging. We need to do more to make researchers and other stakeholders aware of it – while at the same time providing practical recommendations on how to deal with it, such as published examples and methods to assess, minimize and report phototoxicity.
For these recommendations, we need to be realistic about what people are likely to adopt. A very long list of everything that can go wrong, and all the things one should do to make sure it does not, would be overwhelming and counterproductive. Recommending practical and reasonable solutions that do not require excessive additional work will be a much more sensible and useful way to go about it. We hope that such recommendations will help researchers to include the assessment and controls for phototoxicity in their live imaging experiment from the start. We would also strongly encourage reviewers and editors of papers involving live imaging to bear this in mind and offer constructive advice on how to address the limitations on the conclusions that can be drawn from the described live study. This, coupled with better education around the problems of phototoxicity in live imaging, would drive a culture change in the field and hopefully lead to better, more robust and reproducible research.
Stay positive. Well, mostly. To raise awareness of observer effects in fluorescence microscopy, it is of course very useful to cite examples of phototoxicity from other live studies. But rather than hold workshops entitled something like “Death and destruction by fluorescence excitation” or “Irreproducible results in live imaging”, we think it will be more encouraging and productive to have a positive attitude. So we envisage contributions along the lines of ‘best practices in live imaging’. Where possible, this will also include aspects that go beyond phototoxicity, to provide a more comprehensive view of live fluorescence microscopy.
Small is beautiful. Of course we’d say that – we like microscopy! But the point here is that instead of having, for example, an entire conference dedicated to the dangers of phototoxicity, it is more useful to have small contributions that can be set up at short notice and/or consumed with ease. A form of drive-by presentations and Public Service Announcement videos, if you wish. Also, we’re pulling together some useful standard presentation slides for researchers and core facility staff who would like to include the topic in their own presentations. If you’re interested in being part of the solution, please drop us a line and we’ll keep you in the loop (see email addresses at the end).
No size fits all. We’d love to have for example a nifty test sample that we can put under the microscope to reveal whether the levels of excitation light that we are using are safe. However, that is sadly not a realistic solution. Phototoxicity depends on so many different factors: sample type, developmental stage, localization of the fluorescent protein(s) or dye(s), media, wavelength(s), microscope method, image acquisition parameters etc. So there cannot be a single generic way to test for phototoxicity. Individual experimentalists will still need to access any published data available around specific applications, or conduct appropriate studies to identify control experiments which are contextual to the cells, probes and microscopy platforms to be used. But we want to lower the energy barrier for researchers to do this by providing examples, advice and references aplenty.
The reviews cited in this article are a good starting point to think about how you can ensure that your fluorescent sample is imaged in a non-damaging way. Other quantitative studies on phototoxicity have focused on superresolution [4,6,7], compared different imaging techniques [8-10], demonstrated the power of image analysis [11] or the importance of avoiding computer-controlled overhead for exposure times [12]. Recent approaches explore deep learning, either as a tool for assessment [13] or to restore images with very low contrast. The latter can be exploited to reduce the excitation intensity for fluorescent samples [14-17]. In short, there are many different ways that can be used to lower the risk of phototoxicity affecting your sample. We will endeavour to stay updated and include such contributions in our future workshops.
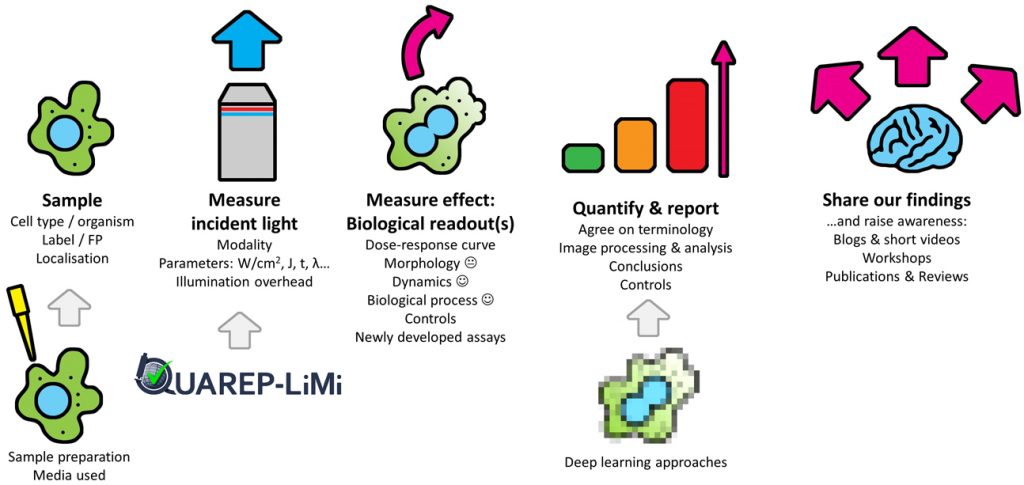
How much light can your sample tolerate? Phototoxicity affecting a sample can be assessed by measuring any change that scales with light intensity; this dose–response curve determines the phototoxicity threshold for any given experimental setup. Accordingly, it is important to damage your sample with light – so you know what to look for. Equally important is finding the best biological readout to assess your sample’s response – the more sensitive a biological readout, the better. Quantifying live cells versus dead ones, or photobleaching, are crude readouts. Morphology is not very sensitive either, as phototoxicity may occur long before morphological changes are visible. Dynamic parameters based on a biological process are far more sensitive approaches, as is checking for long-term damage after the experiment is finished. A particular challenge is however posed by very short-term experiments (seconds to minutes), where long-term readouts (such as mitotic delay) may not be available or practical.
We’ve got the power. Not so long ago, an editorial [18] stated that “the time is right for developers and biologists to seek a more quantitative understanding of the effects of light on samples.” We couldn’t agree more. But exactly how much light triggers a response in a given sample? Power measurements are fundamentally important if we want absolute quantification of phototoxic effects on a given sample. New methods are being developed, while other approaches that allow for user-friendly, highly convenient intensity measurements, such as the Meta-Max [19], are in their fourth round of improvement. It is important to note that power measurements alone cannot replace an assay of biological activity for your particular sample to check for phototoxicity – see also ‘no size fits all’ above.
Together we’re better. Although we have focused on phototoxicity, the goal ultimately is to reduce artefacts and improve discovery. This is congruent with the remit of the QUAREP-LiMi initiative, which stands for ‘Quality Assessment and Reproducibility for Instruments & Images in Light Microscopy’. QUAREP-LiMi is a global group of enthusiastic stakeholders including imaging scientists from academia and industry that aim to improve reproducibility for light microscopy image data through QC management of instruments and images. QUAREP-LiMi is currently focused on the technical QC of the instruments rather than the user-controlled experimental details such as dyes and samples. However, maintaining close contact with QUAREP-LiMi will be important to ensure QC checks are aligned with experimental guidance to avoid toxicity problems in live imaging. Well-calibrated light sources and detectors will be of foundational importance to comprehensively and robustly quantify phototoxic effects. However, we also believe that it is never too early to start raising awareness.
Can we have a bigger table please? It is important to widen the discussion. Our first meeting consisted of researchers, ranging from core facility staff to group leaders, but we need to broaden the discussion to all stakeholders. So as well as researchers, trainees and imaging facility staff, we want scientific publishers, reviewers of manuscripts and grant applications, and optical instrument manufacturers to join our round table. We want this to be a strong, inclusive, comprehensive and solutions-focussed community.
For now, we have clear intentions, enthusiasm and a feasible plan to start an educational program on best practices in live imaging. However, getting funding for this kind of initiative has proven difficult for many participants. Finding sponsors for studies on phototoxicity and workshops on live imaging will be important in the longer run.
Contact: If you are interested in participating in workshops on phototoxicity (challenges and guidance) and ‘Best Practices for Live Imaging’, or would like to inquire about the availability of these, please contact Philippe Laissue or Claire Brown.
Next opportunity: Dr Claire Brown and myself will be running a free workshop on phototoxicity on Friday 9 July 2021 (14.00 – 17.00 hours BST) during the upcoming Microscience Microscopy Congress 2021.
Acknowledgements: We would like to thank all the speakers and participants for their invaluable contributions: Joerg Bewersdorf (Yale University, US), Robert Campbell (University of Alberta, CA), Youssef Chebli (McGill University, CA), Teng-Leong Chew (Janelia Research Campus, US), Siân Culley (University College London, UK), Davide Danovi (King’s College London, UK), Anja Geitmann (McGill University, CA), Laurent Gelman (Friedrich Miescher Institute, CH), Campbell Gourlay (University of Kent, UK), David Grunwald (University of Massachusetts Medical School, US), Alex Laude (University of Newcastle, UK), Anne McKinney (McGill University, CA), Dan Mulvihill (University of Kent, UK), Glyn Nelson (University of Newcastle, UK), Alison North (The Rockefeller University, US), Raghuveer Parthasarathy (University of Oregon, US), Emmanuel G. Reynaud (University College Dublin, IE), Hari Shroff (National Institute of Biomedical Imaging and Bioengineering, US), Jennifer Waters (Harvard Medical School, US), Tanya Whitfield (University of Sheffield, UK).
Organisers:
Claire Brown, McGill University, CA
Deirdre Kavanagh, COMPARE, University of Birmingham, UK
Philippe Laissue, University of Essex, UK
Chas Nelson, University of Glasgow, UK
Maddy Parsons, King’s College London, UK


 (No Ratings Yet)
(No Ratings Yet)