Hot reads
Posted by FocalPlane, on 5 October 2021
Our monthly post is back. We have asked different researchers to propose their favourite recently published papers (related to microscopy, of course).
These are the suggestions for this month. We hope you find some interesting publications that were not included in your reading list.
Department of Molecular and Cellular Physiology, Albany Medical College, Albany, NY (USA)
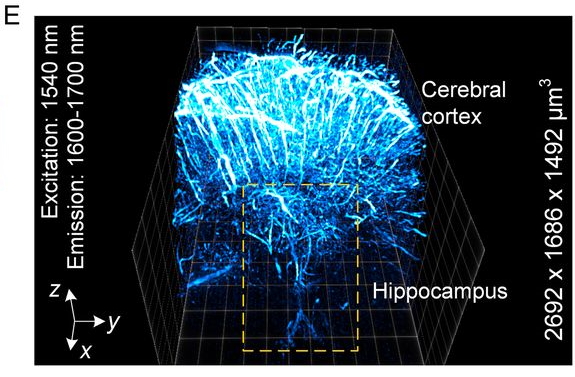
- ‘In vivo NIR-II structured-illumination light-sheet microscopy’ https://doi.org/10.1073/pnas.2023888118 A noninvasive optical imaging technique with deep tissue penetration depth and high spatiotemporal resolution
- ‘Automated segmentation and tracking of mitochondria in live-cell time-lapse images’ https://doi.org/10.1038/s41592-021-01234-z Mitometer, an unbiased and user-friendly tool for segmenting and tracking mitochondria in 2D and 3D time series.
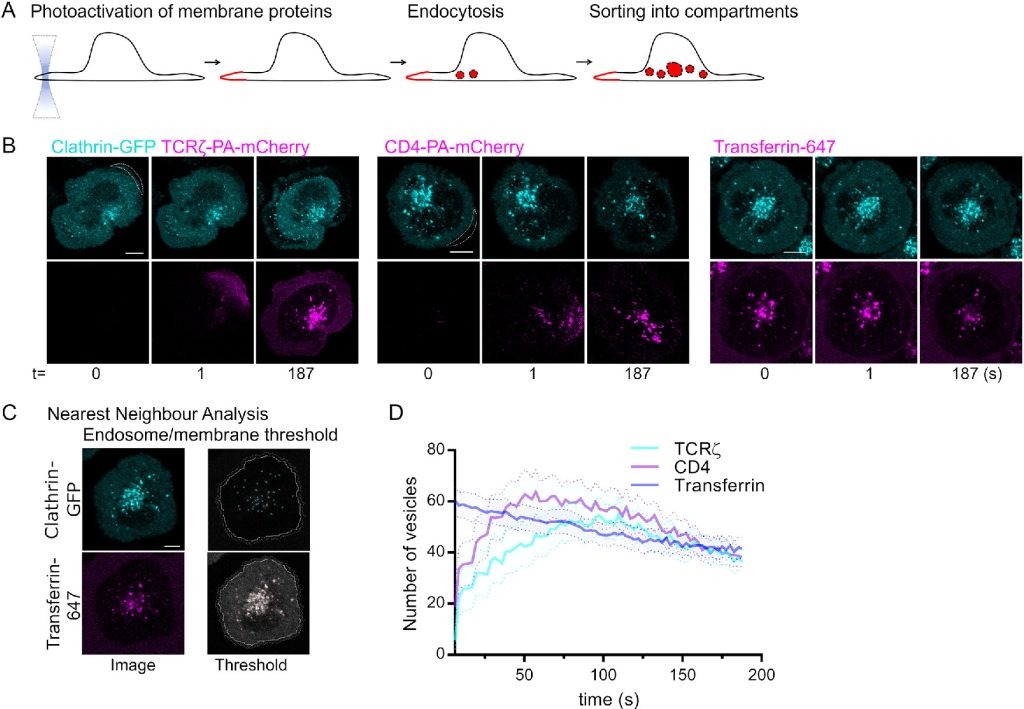
- ‘Quantitative visualisation of endocytic trafficking through photoactivation of fluorescent proteins’ https://doi.org/10.1091/mbc.E20-10-0669 A toolset based on photoactivation of fluorescent proteins that enables quantification of endocytosis.
- ‘pHmScarlet is a pH-sensitive red fluorescent protein to monitor exocytosis docking and fusion steps’ https://doi.org/10.1038/s41467-021-21666-7 A bright red-shifted pH sensitive fluorescent protein allows following all steps of vesicle exocytosis.
CellComM Lab- IBMZ, University Medical Center Hamburg- Eppendorf | UKE
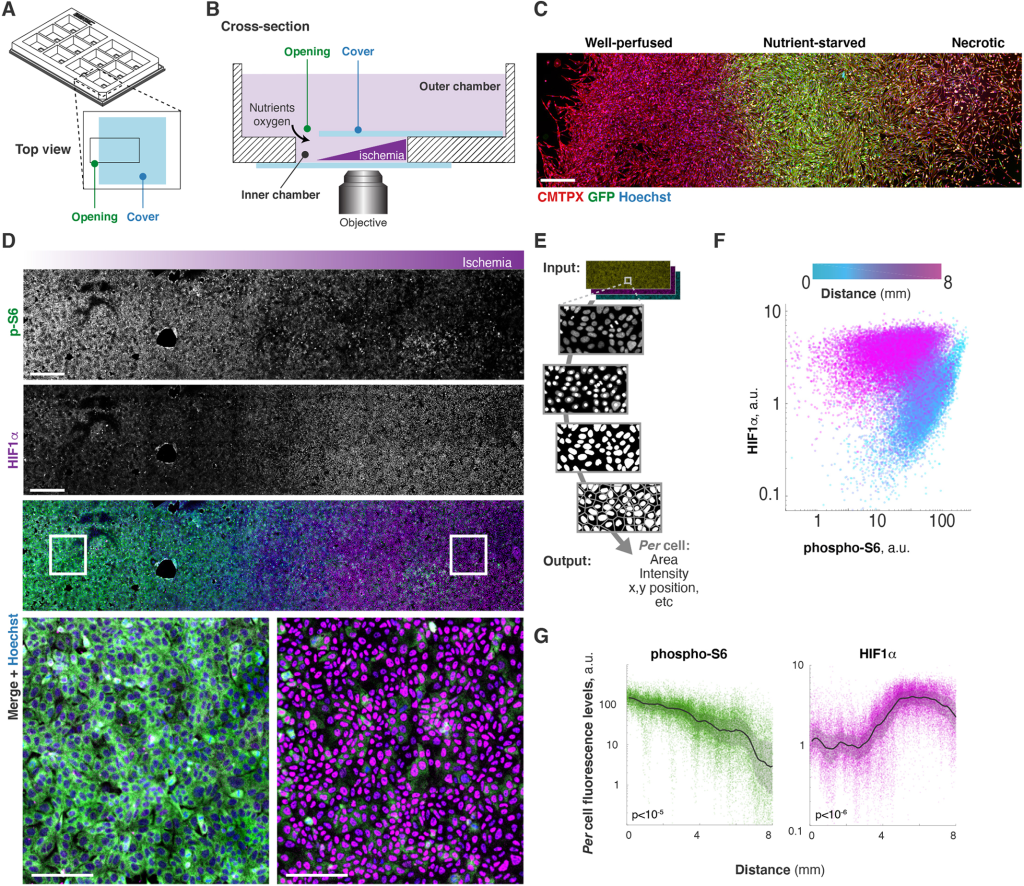
- “The MEMIC is an ex vivo system to model the complexity of the tumor microenvironment” https://doi.org/10.1242/dmm.048942 This papers contains a very good description of MEMIC, a 3-D printed chamber that allows live-cell imaging the development of metabolic conditions found in the tumoral microenvironment. In the authors words MEMIC is ‘a microphysiological system’.
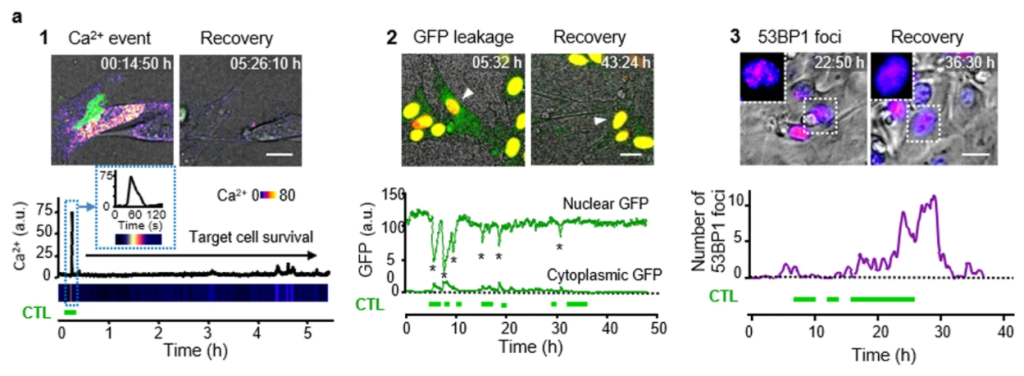
- “Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity” https://doi.org/10.1038/s41467-021-25282-3 In this article the authors use in vitro and in vitro live cell imaging to monitor calcium signaling, leukocyte migration and interaction with cancer cells to show that cytotoxic T cells induce sublethal damage, which over time is accumulated and lead to the cancer cell death. Therefore, the cytotoxic T cell killing capacity depends on the number and cooperation of these cells.
- “Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion” https://doi.org/10.1016/j.cell.2021.08.035 This papers combines different techniques including microfluidics, live-cell imaging, and imaging analysis to show that the confinement deforms the nucleus damaging the DNA, which leads cancer cells to be more invasive. The same is found in human tumors validating the in vitro and in vivo (mice) results.


 (No Ratings Yet)
(No Ratings Yet)