Tumors want to break free! How knocking down Myo10 can change invasive properties of a breast cancer xenograft model.
Posted by FocalPlane, on 16 December 2021
This is one of the research highlights from our second preLights – FocalPlane social writing event. You can find the other reseach highlight in preLights.
Myosin-X-dependent assembly of the extracellular matrix limits breast cancer invasion
Emilia Peuhu, Guillaume Jacquemet, Colinda LGJ Scheele, Ilkka Paatero, Kerstin Thol, Aleksi Isomursu, Maria Georgiadou, Camilo Guzman, Satu Koskinen, Asta Laiho, Laura L Elo, Pia Bostrom, Pauliina Hartiala, Jacco van Rheenen, Johanna Ivaska
https://www.biorxiv.org/content/10.1101/2021.10.22.464987v1
Background/Introduction
Breast cancer is the most commonly diagnosed cancer in women (1). Advances in its clinical management have been achieved due to experimental models which recapitulate several features of disease progression (2). The MCF10 series of cell lines is one such system which has contributed to seminal discoveries in breast cancer research (3). MCF10DCIS, a subline derived from the parental MCF10 cells, is often used to model the in situ to invasive breast carcinoma transition and has been used in this preprint to offer further insights into this process.
Tumor invasion is a complex phenomenon that involves multitudes of changes in tissue architecture and cancer cell behavior. Phenotypic changes in tumor cells allow them to migrate and break free from the primary tissue by degrading the underlying matrix. Recent studies have suggested the generation of stiff-ECM invasive tracks for tumor cells (4-6). Studies demonstrate how tumor cells establish novel interactions with these ordered ECM structures and enhance their invasive capacities (7,8). This initiates the metastatic cascade which enables distant seeding of tumor cells and is a clinical complication in patients presenting with advanced-stage disease. Hence, understanding how tumor invasion works by reorganizing ECM structures may help in the development of therapies that curb this process.
In this preprint, the authors use the MCF10DCIS cell system to understand the contribution of a molecular motor, MYO10, to tumor invasion. Using knockdown xenograft models, they demonstrate how MYO10 depletion generates a more invasive tumor by virtue of changes in ECM deposition. Importantly, the authors suggest a compensatory mechanism for ECM synthesis which is driven by MYO10 knockdown.
Key Findings
MYO10 knockout affects tumor cell invasion
The authors observed MYO10 expression in clinical samples of pre-invasive human ductal carcinoma in situ (DCIS). To examine the role of this molecular motor in cancer progression, they knocked down MYO10 in MCF10DCIS cells. Xenograft tumors from this cell system have been previously characterized, which allowed the authors to study tumor invasion at specific time points. While MYO10 knockdown cells exhibited slower migration and used lamellipodia rather than filopodia for this process, tumors generated from these cells were more invasive in nature. This observation was further supported by the higher frequency of mesenchymal cells in the knockdown tumors and the increased distribution of tumor cells along the basal lamina.
MYO10 knockout alters the expression of ECM-associated genes in cancer cells
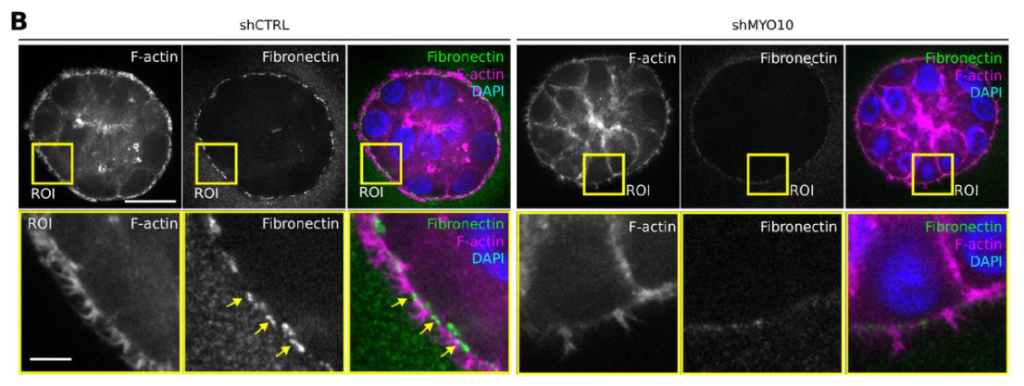
As breaking through the basement membrane (BM) is a crucial step during cancer progression, the authors examined BM composition in xenograft tumors. They achieved this by detecting the deposition of extracellular matrix (ECM) proteins, collagen IV and fibronectin, which were almost absent in knockdown tumors. The authors further confirmed changes in ECM deposition by using tumor spheroids. MYO10 knockdown resulted in reduced accumulation of fibronectin and collagen IV at the boundaries and intercellular junctions of the spheroid (Figure 1). Surprisingly, a bulk RNA sequencing experiment identified the enrichment of ECM-related genes in the knockdown tumors. The authors propose that this could result from a possible compensatory mechanism whereby the tumor cells may produce more ECM to facilitate invasion.
Why is this paper interesting?
Models for cancer progression are a valuable tool to understand the molecular and cellular contributors of this disease. In this preprint the authors use a well-characterized xenograft model of ductal carcinoma in situ to decipher how MYO10 governs tumor cell invasion by changing cell membrane extrusions and ECM deposition. The authors leveraged their observations with intra-vital imaging, high resolution confocal and electron microscopy approaches and provided insights into the tumor cell-ECM interactions at the basement membrane. Observations from this study hold relevance in understanding the initial stages of cancer invasion and further accentuate our knowledge pertaining to ECM deposition at the tumor edge.
Questions to the authors
- How does switching the cellular extrusions from filopodia to lamellipodia affect disease progression? From a future perspective, do you intend to examine changes in the migratory modalities of the knockout cells?
- Does MYO10 knockout alter the phenotypic plasticity of your model system by influencing epithelial-mesenchymal transitions?
- Do you think that some of the observations pertaining to BM integrity may arise from changes in its ECM constituents as opposed to a complete loss of the ECM?
References
1. Siegel RL, Miller KD, et al. Cancer Statistics, 2021. CA: a Cancer Journal for Clinicians. 2021 Jan;71(1):7-33. DOI: 10.3322/caac.21654. PMID: 33433946.
2. Roarty K, Echeverria GV. Laboratory Models for Investigating Breast Cancer Therapy Resistance and Metastasis. Front Oncol. 2021;11:645698. doi: 10.3389/fonc.2021.645698.
3. Puleo J, Polyak K. The MCF10 Model of Breast Tumor Progression. Cancer Res. 2021 Aug 15;81(16):4183-4185. doi: 10.1158/0008-5472.CAN-21-1939. PMID: 34400468.
4. Nicholas Boluda A, Vaquero J, et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife. 2021 Feb; DOI: https://doi.org/10.7554/eLife.58688.
5. Krajina BA, LeSavage BL, et al. Sci. Adv. 2021; 7:eabe1969;
DOI: 10.1126/sciadv.abe1969.
6. Matte BF, Kumar A, et al. Matrix stiffness mechanically conditions EMT and migratory behavior of oral squamous cell carcinoma. 2019 Dec; 132, J. Cell. Sci. doi:10.1242/jcs.224360.
7. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020 Oct 9;11(1):5120. doi: 10.1038/s41467-020-18794-x. PMID: 33037194; PMCID: PMC7547708.
8. Xiong GF, Xu R. Function of cancer cell-derived extracellular matrix in tumor progression. J Cancer Metastasis Treat. 2016;2:357-64 . http://dx.doi.org/10.20517/2394-4722.2016.08.
Author’s response
- How does switching the cellular extrusions from filopodia to lamellipodia affect disease progression? From a future perspective, do you intend to examine changes in the migratory modalities of the knockout cells?
We previously found that targeting filopodia in an aggressive breast cancer model decreases cancer cell migration and metastasis. In this manuscript, we found that at an earlier stage of the disease, silencing of MYO10 compromises basement membrane assembly and promotes local dissemination most likely owing to the initially defective generation of the basement membrane barrier surrounding the MYO10 silenced tumors.
So, to answer the question, we would hypothesize that switching from filopodia to lamellipodia may be linked to defective basement membrane assembly accelerating disease progression and local invasion in the early stages of breast cancer. However, given the role of filopodia in invasion, switching of the migration mode may limit tumor metastasis to distal sites.
We are indeed currently studying in more detail how MYO10 filopodia contribute to the collective cell migration behavior of DCIS.com cells both in vitro and in vivo! Stay tuned for more on this in the coming years.
- Does MYO10 knockout alter the phenotypic plasticity of your model system by influencing epithelial-mesenchymal transitions?
This is an excellent question. At this stage, it remains unclear if the increased frequency of mesenchymal cancer cells at the border of MYO10 depleted tumors is a direct consequence of MYO10 silencing or indirectly caused by the compromised basement membrane. However, in vitro, we could not detect any differences in vimentin or E-cadherin protein levels upon in MYO10 silencing and experimental EMT-induction with TGFbeta, suggesting the latter.
- Do you think that some of the observations pertaining to BM integrity may arise from changes in its ECM constituents as opposed to a complete loss of the ECM?
This is indeed a possibility. So many open questions remain on how basement membranes are organized and how their properties change in different conditions and compositions. However, in the case of MYO10 depleted tumor, it looks like a remodeling/formation issue. This is very exciting, as basement membranes have typically been seen as static barriers, and very little is known about their turnover/homeostasis.





 (No Ratings Yet)
(No Ratings Yet)