Illuminating Cancer Metabolism: Unveiling Mitochondrial Secrets Through Fluorescence Microscopy
Posted by SANGRAM POLLEY, on 23 March 2025
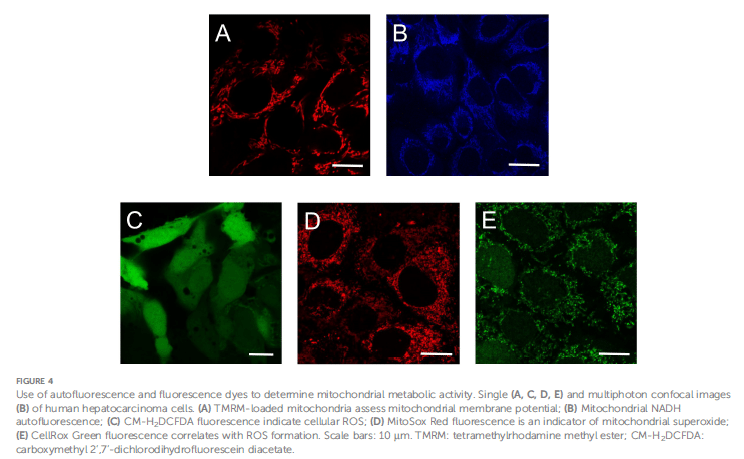
Understanding cancer metabolism is central to developing effective therapies, and a chief player in this complex scenario is mitochondrial metabolism. The studies of Monika Gooz and Eduardo N. Maldonado have shed light on how fluorescence microscopy is employed to investigate mitochondrial activity in cancer cells, thus posing avenues into the metabolic vulnerabilities that could be tackled for therapeutic favors. Mitochondrial metabolism is much aligned with cancer cell survival and proliferation because it holds much in the way of ATP synthesis, reactive oxidative species (ROS) homeostasis, and biosynthetic activities. While the Warburg effect predominantly emphasizes glycolysis as the energy source for cancer, there appears to be a delicate interaction in which oxidative phosphorylation (OxPhos), until recently somewhat disregarded in the aggressive form of cancers, maintains its relevance. Fluorescence microscopy has become essential for resolving this paradox, enabling high-resolution imaging of mitochondrial membrane potential (ΔΨm), nicotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide (FAD), ATP, and ROS. These methods include widefield, confocal, and multiphoton microscopy, all of which have facilitated the visualization and quantification of metabolic indicators by using spatiotemporal resolution; FLIM adds another layer of information by measuring the decay time of fluorescence in tandem with changing conformational states of the molecules. Nevertheless, widefield microscopy suffers from having a great deal of background noise and reduced resolution, which often includes deconvolution. In contrast, confocal microscopy does a much better job because confocal microscopy dispenses with the out-of-focus light through the use of a pinhole, producing much higher contrast and resolution. Multiphoton microscopy uses longer wavelengths in the infrared region that penetrate deeper into tissues with the least photodamage for imaging NADH and FAD auto-fluorescence. The technically skillful methods of FLIM distinguish favorable fluorescence decay times that reflect free or protein-bound NADH and hence are vital for elucidating cellular redox states. In addition to imaging techniques, fluorescent probes and biosensors are of great importance in the quantification of mitochondrial activity. Potentiometric dyes like TMRM and ratiometric dyes such as JC-1 are used more commonly to measure ΔΨm, while genetically encoded biosensors allow tracking of ATP and NADH in real time. Specifically, SoNar and Frex are biosensors that have provided exceptional measurements of NadH/NAD+ ratios in the cytoplasm and mitochondria, respectively, which contribute greatly to understanding metabolic flux. A noteworthy finding was advanced by fluorescence-based imaging of ROS produced by mitochondrial complexes I and III, which brings to light the importance of oxidative stress in cancer. MitoSox Red and CM-H2DCFDA probe the production of superoxide and hydrogen peroxide, respectively. Genetically encoded sensors like Hyper and MitoHyPer continue to provide the most accurate estimate of H2O2 dynamics in cellular compartments.
Decoding Mitochondrial Metabolism in Cancer: The Role of Fluorescence Microscopy
Mitochondrial metabolism has acquired a significant role in sustaining life and replication of the cancerous cells, in cooperation with a high rate of glycolysis. The Warburg effect, defined first by Otto Warburg, considered that cancer cells favored aerobic glycolysis in the presence of oxygen, although more recent studies have shown mitochondria also provide active support for tumor growth. Understanding the fine balance between glycolysis and oxidative phosphorylation (OxPhos) in cancer cells may lead to the identification of metabolic weaknesses, which could be advantageous in therapy. Fluorescence microscopy, one of the most invaluable imaging approaches, provides tools for studying mitochondrial bioenergetics in cancer cells through semiquantitative and quantitative readouts having exceptional spatial and temporal resolution.
The mitochondria function as “powerhouses” of the cell, oxidizing substrates like pyruvate, fatty acyl-CoAs, and some amino acids in the Krebs cycle, producing NADH and FADH2 to enter the electron transport chain (ETC). This generates a proton gradient across the mitochondrial inner membrane, which establishes a mitochondrial membrane potential (ΔΨm) that ultimately facilitates ATP synthesis through oxidative phosphorylation. But alongside ATP production, its by-products, ROS, also determine cell fate. Fluorescence microscopy will enable the real-time visualization and measurement of key metabolic indicators like ΔΨm, NADH, FAD, ROS, and ATP with enhanced sensitivity, allowing the further identification of the metabolic alterations involved in cancer progression and therapeutic response.
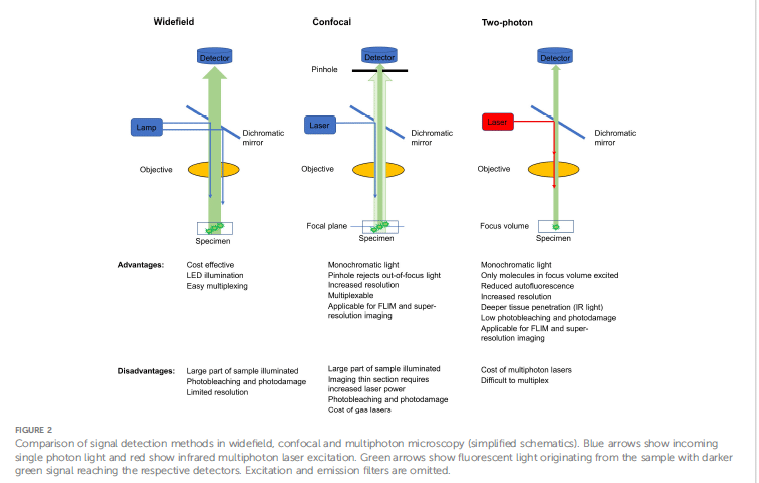
Widefield, Confocal, and Multiphoton Microscopy: A Comparative Lens
In terms of commonly used imaging modalities, widefield microscopy provides an economical technique for detecting fluorescent signals in live cells. Its drawbacks, including greater levels of background noise and diminished resolution, give further weight to the justification for applying image deconvolution algorithms to hold contrast enhancement. Confocal microscopy is based on the use of a pinhole that blocks light emanating from outside the plane of focus and thus provides better resolution and contrast, rendering itself nice to visualize mitochondrial structures and metabolic changes. Multiphoton microscopy, on the other hand, uses infrared light to excite endogenous and exogenous fluorophores and can penetrate deep into tissues with less photodamage. Hence it is the technique of choice for NADH and FAD autofluorescence imaging as it minimizes photobleaching allowing researchers to follow dynamic changes in cellular metabolism with utmost sensitivity.
Fluorescence Lifetime Imaging Microscopy (FLIM): A Window into Cellular Redox States
FLIM stands for fluorescence lifetime imaging, refers to an advanced imaging platform, which reports the time spent by fluorophore in the excited state before returning to the ground state—a unique fingerprint for particular molecular interactions. This technique should aid in distinguishing between free and protein-bound NADH most accurately while assessing redox state in cells. Since NADH fluoresces with a different lifetime depending on its bound state, FLIM allows us to monitor the metabolic state of cancer cells by detecting the metabolic shifts during tumor progression, metastasis, and therapeutic response.
Probes, Dyes, and Biosensors: Illuminating Mitochondrial Dynamics
To investigate mitochondrial metabolism of cancer cells, a plethora of fluorescent probes, dyes, and biosensors have gained prominence. Potentiometric dyes such as TMRM and rhodamine 123 accumulate in mitochondria based on ΔΨm, facilitating the use of either mitochondrial visualization and the assessment of functional changes. Ratiometric dyes offer quantitative measurements of the mitochondrial membrane potential, whereby JC-1 transitions from green to red fluorescence with increasing ΔΨm. In a similar manner, fluorescent biosensors Frex, FrexH, and SoNar permit the real-time assessment of NADH and NAD+/NADH ratios providing dynamic information on mitochondrial function. Genetically encoded sensors estimation of H2O2 dynamics in cellular compartments such as MitoHyPer and HyperRed allow monitoring oxidative stress in live cells.
Reactive Oxygen Species (ROS): A Double-Edged Sword in Cancer Progression
Reactive oxygen species (ROS) produced in mitochondria, mainly through the action of complexes I and III of the electron transport chain (ETC), can prove beneficial or harmful to cancer progression. Exposure to low levels of ROS contributes towards signaling and survival, while excessive ROS can cause mitochondrial injury and initiate apoptosis via oxidative injury. Fluorescent probes such as MitoSox Red and CM-H2DCFDA are able to incorporate the visualization and quantification of mitochondrial superoxide and hydrogen peroxide production sources. Hence, they can help match generation to antioxidants and light a difficult balance concept. The most exciting area here is probably the emerging development of mitochondria-targeted antioxidants such as Mito-Q and Mito-Tempo to counter oxidative stress in cancer therapy.
Applications in Cancer Research and Therapy: A Path Toward Personalized Medicine
These bring fluorescence microscopy into tumor metabolism and offer the potential for individualized therapies. There is now an area of high-content imaging platforms that allow one to carry out large scale screenings of chemical libraries for identifying new metabolic targets. On the other end, mitochondria heterogeneity was quantified with the intention of understanding the tumor microenvironment better. But fluorescence imaging shows another kind of promise, distinguishing normal from precancerous to cancerous tissues, in that it forms a non-invasive monitor of tumor behavior in prediction of outcome of therapies. Multiphoton imaging as well as FLIM application were directed at determining metabolic status of immune cells in the tumor microenvironment for developing immunotherapeutic strategies.
Challenges and Future Directions: Toward Greater Precision in Metabolic Imaging
Fluorescence microscopy is powerful to transform cancer research but remains to face appreciable challenges yet to be solved. Improving the specificity of the probe, reducing phototoxicity, and making mitochondria-dye-specific with lower off-target effects remain ongoing avenues for investigation. Meanwhile, the heterogeneous view of mitochondrial metabolism in tumors emphasizes the need for quantitative imaging methodologies that diversify cellular information. As fluorescence microscopy becomes ever more refined, the avenues available for cancer diagnostics and treatment promise to shed light on metabolic vulnerabilities for precision targeting. In the light of advanced imaging methods and biosensors, the way is wide open to disentangle the enigma of cancer metabolism for improving efficacy and personalized management of the patients.


 (3 votes, average: 1.00 out of 1)
(3 votes, average: 1.00 out of 1)