Volume EM Visualization of 3D Synaptic Ultrastructure in Postmortem Human Prefrontal Cortex
Posted by Janithri Wickramanayake, on 31 October 2025
by J.R. Glausier1, M. Maier1, J. Kozel1, C. Bouchet-Marquis2, K. Wu2, et al.,
1University of Pittsburgh and 2Thermo Fisher
Challenge
Synaptic function and ultrastructure are intrinsically coupled; thus, volume electron microscopy (vEM) offers the ability to quantitatively interrogate multiple ultrastructural correlates of function within the individual synapses that populate a brain volume. By applying vEM to human brain tissue, synaptic properties that contribute to complex behaviors, and their disruption in disease, can be directly interrogated. However, vEM studies of human brain tissue obtained postmortem are associated with unique challenges that pose obstacles to achieving the high-contrast, ultrastructurally-intact tissue required to generate quantitative vEM datasets. For example, these tissues are often associated with a postmortem interval (PMI) and tissue sections may be stored for extended periods before vEM sample preparation.
To help overcome these obstacles, we optimized a tissue processing workflow for focused ion beam-scanning electron microscopy (FIB-SEM) imaging of postmortem human brain tissue. This workflow was validated in prefrontal cortical tissue samples with a 6-hour PMI and that were stored for ~8 years before vEM sample preparation.
Technique: HVYMTL Staining
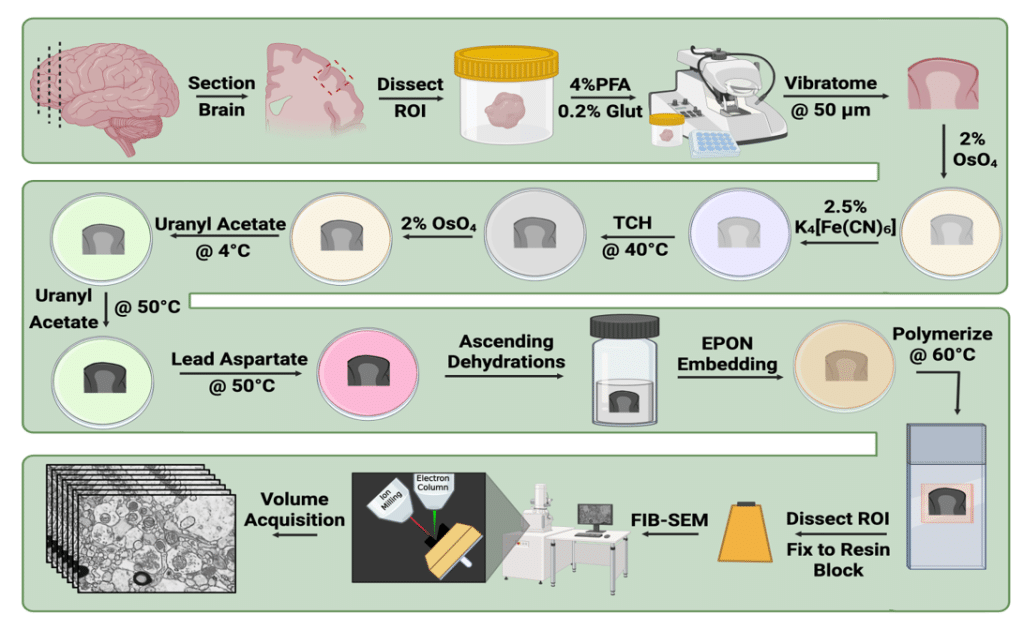
Figure 1. Heavy metal (HVYMTL) sample preparation workflow using postmortem human prefrontal cortical samples. The Hua et al. 2015 approach was modified to account for notable differences in tissue extraction, fixation, sampling, and long-term storage conditions common for postmortem human brain tissue. Our protocol reliably generated brain tissue samples suitable for FIB-SEM imaging.
Research
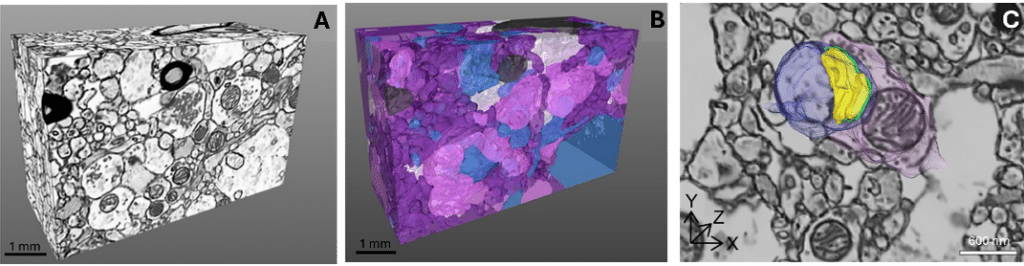
Figure 2: Representative examples of 3D volumes and reconstructions. (A) Postmortem human cortical neuropil volume imaged in 3D via FIB-SEM. (B) Corresponding dense 3D reconstruction of all neuronal and glial profiles shown in (A). Glutamate axonal boutons (pink), dendritic shafts and spines (blue),unmyelinated axons (purple), myelinated axons (dark gray), and astrocytic processes (white). (C) Reconstructed Type I synapse shown within the context of the surrounding neuropil: presynaptic axonal bouton (pink, 0.93 mm3), postsynaptic dendritic spine (blue, 0.50 mm3), active zone (green, 7.1e6 nm3), postsynaptic density (yellow, 16.8e6 nm3).
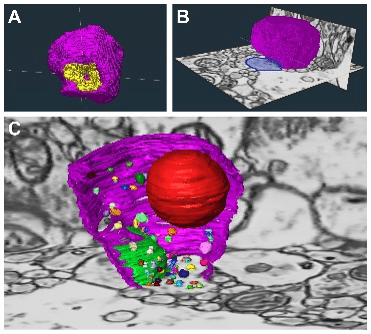
Figure 3. 3D segmentation and reconstructions of glutamate synaptic and sub-synaptic structures in postmortem human cortex. (A) Relationship between the axon terminal (pink) and PSD (yellow), which is perforated. (B-C) Synaptic and sub-synaptic reconstructions shown in the context of the surrounding tissue. Dendritic spine (blue), active zone (green), synaptic vesicles (multi) and mitochondrion (red).
Impact
• FIB-SEM analysis of postmortem human brain tissue is feasible with HVYMTL staining and can yield high-quality volumetric datasets capable of supporting detailed, quantitative ultrastructural analysis.
• Provides proof-of-concept that long-archived postmortem human brain tissue samples may be suitable for systematic and quantitative vEM studies of human brain disorders such as schizophrenia and Parkinson’s disease.
References
Hua Y, Laserstein P & Helmstaedter M. Large-volume en-bloc staining for electron microscopy-based connectomics. Nat Commun 6, 7923 (2015). https://doi.org/10.1038/ncomms8923
Glausier JR, et al. Volume electron microscopy reveals 3D synaptic nanoarchitecture in postmortem human prefrontal cortex. iScience 28, (2025). DOI: 10.1016/j.isci.2025.112747
Poster



 (No Ratings Yet)
(No Ratings Yet)