A new Review in Journal of Cell Science discusses how specimens can impact image acquisition
Posted by Helen Robertson, on 13 April 2022
With the development of new imaging strategies and technologies and advanced computational analyses has come a body of literature addressing various aspects of optimising imaging and processing. However, the impact of the sample itself on the quality of the image attained has garnered less exploration. Fluorescent microscopy is dependant on two factors – the intensity of the signal, and the location of the signal – for experimental readout. That means anything within the sample itself that perturbs one, or both, of these values will result in an inaccurate reflection of the specimen being studied.
To try and address specimen-derived problems in fluorescent microscopy, a team of researchers from Janelia Research Campus have written a recent Review for Journal of Cell Science (JCS). In their article ‘When light meets biology – how the specimen affects quantitative microscopy’, the authors outline the main sources of specimen-based problems in quantitative analysis in microscopy and provide advice on how to correct for these errors in experiment design and image analysis. Dr Michael Reiche, Advanced Microscopy Imaging Fellow at Janelia Research Campus and first author of the Review said, “The main motivation behind this review was to highlight the biological complexities inherent to fluorescence microscopy experiments. Bioimaging really does lie at the convergence of physics, chemistry, data science, and biology; unfortunately, the latter is often and inadvertently overlooked. Appropriately interpreting data is just as important as correctly capturing it”.
Here, we provide a highlight of some of their discussion. For further information and additional resources, we highly recommend reading the Review, available in the recent issue (6) of JCS.
Identifying and reducing phototoxicity
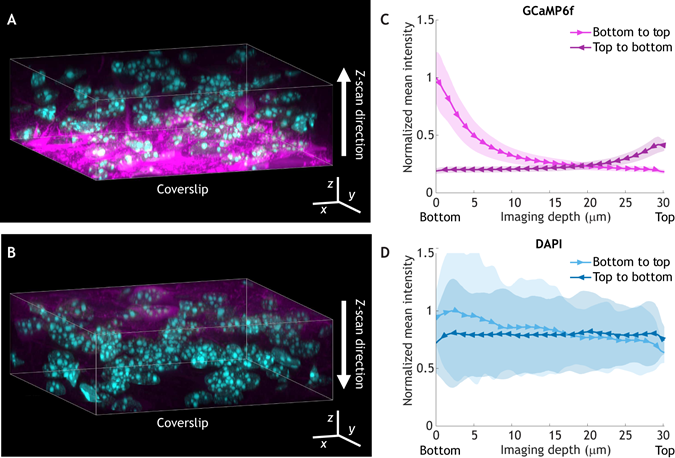
As the authors describe, the high illumination intensity required for fluorescent microscopy can be damaging to the sample itself, with various consequences including decreased cell proliferation, mitochondrial fragmentation, and in extreme cases, cell death and destruction of the sample. So, how do you measure phototoxicity – and how do you correct for the affect of phototoxicity in designing your experiment? Time-lapse experiments are a useful way of assaying photosensitisation: compare readouts throughout the course of the experiment with those imaged at the end of the experiment or with different experimental parameters to identify differences in the sample as a result of light exposure.
Usefully, the authors describe a number of studies with suggestions for how to reduce phototoxicity. These include using the lowest level of illumination possible, the use of bright and photostable fluorophores, and being mindful of the environment in which you image your sample to minimise the presence of reactive oxygen species (ROS).
Autofluorescence
Autofluorescence occurs as a result of the endogenous fluorescence capacity of biological molecules. For anyone who has tried to image cnidarians – from which GFP is derived – you’ll know the problems that strong endogenous autofluorescence can pose to accurately measuring your signal! But there are useful steps you can take to reduce the impact of autofluorescence on your image acquisition. Firstly, make sure to select fluorophores that emit in the far-red region, as autofluorescence tends to occur in the green spectrum. Spectral unmixing approaches can also help discriminate between the signal-of-interest and autofluorescence – which many confocals can do by separating out the wavelengths of light detected, allowing autofluorescence to be isolated computationally. If the signal-of-interest has an overlapping wavelength with autofluorescence, the authors recommend separating out molecules based on their fluorescent decay – achieved by using fluorescence lifetime imaging microscopy (FLIM).
Sample microenvironment
The accuracy of your image is not just impacted by your specimen: the microenvironment of the cell can also alter bioimage acquisition. Many variables in the microenvironment can impact fluorophore behaviour, including pH, redox state (contributing to the presence of ROS) and voltage potential, and the authors cite several papers describing the impact of these variables on biological accuracy of the acquired image. Whilst overcoming these factors is not always easy, the authors recommend consulting with the manufacturer of the fluorescent probe to identify its characteristics and the optimal environment for its use, as well as literatures searches for tried-and-tested experimental application and designing appropriate controls to assay variation in signal.
In summary
Whilst this is just a brief introduction to the useful advice provided in the review, it is clear that optimising sample preparation and experiment design is central to the biological fidelity of the images you acquire. Of course, this is not a straightforward task. As Michael says, “The caveat and difficulty is that an object and its image are not the same thing. There will always be some degree of deviation. The key is understanding how the discrepancy between the ‘true’ biology and the captured image might change the accuracy or validity of a particular result”.
Read the full Review for more helpful tips and to find literature recommendations from the authors, and thanks to Michael for providing further insight into the work!
Related reading from JCS
When light meets biology – how the specimen affects quantitative microscopy


 (1 votes, average: 1.00 out of 1)
(1 votes, average: 1.00 out of 1)