Using Nile Blue and Nile Red to visualise the presence of lipids in cryosectioned tissues
Posted by Stuart Fraser, on 14 September 2021
Lipids are crucial elements of mammalian (and non-mammalian) cell biology and yet lipids are challenging to visualise in situ. In comparison to proteins, which we can generate antibodies for, or carbohydrates, some of which we can detect using fluorescent lectins, there are relatively few lipid-specific fluorescent probes. Many lipids are highly conserved across species making the production of antibodies against specific lipids very challenging.
We recently published the use of Nile Blue, a lipophilic dye, in combination with its derivative Nile Red, for the detection of free fatty acids and triglycerides in cells for imaging and flow cytometry (Boumelhem et al., J Cell Sci (2022) 135 (5): jcs258322. https://doi.org/10.1242/jcs.258322). This document aims at being a “users guide” for using Nile Blue and Nile Red for fluorescent cell imaging. Both Nile Blue and Nile Red fluoresce in the presence of lipids in multiple species. We have used both probes to stain cells from rodents, human cancer cell lines, marsupial cells and even planaria (data not shown).
What are these Nile dyes?
Nile Blue was first reported in 1896 though it may have been invented as early as 1888. Nile Blue is an oxazine, blue-coloured, fluorescent histological stain. Its lipophilic properties have been known since 1908 (Lorraine, J., 1908. J Path. Bact. Vol 12, (1), 1-4 https://doi.org/10.1002/path.1700120103). Boiling Nile Blue with sulphuric acid results in Nile Red, a lipophilic stain distinct from Nile Blue both in terms of lipids sensed and fluorescent properties. Both dyes change in fluorescent properties according to the presence of lipids in their local microenvironment rather than binding directly to the lipids. Nile Red is highly solvatochromic, meaning the wavelength of emitted fluorescent light is dependent upon the solvent, and particularly the polarity of the solvent, that Nile Red is dissolved in. This means that Nile Red can be used as an indicator of local polarity such as the polarity on the surface of nanoparticles for example. Both Nile Blue and Nile Red are relatively cheap compared to other fluorescent lipophilic probes. Both are relatively straightforward in terms of storage and solvent choice though as mentioned they can vary in fluorescent properties depending on the solvents used for reconstitution. We typically make a stock solution of Nile Blue in distilled water and a stock solution of Nile Red in DMSO. Both can then be diluted in PBS to use with cells.
Nile Red has a broad range of emission and can be excited using the 488 nm or 565 nm lasers. Nile Blue is a far-red dye and is excited at 625 nm. Typically, Nile Red fluorescent signal in lipid droplets is observed in the orange-red channels whereas Nile Blue fluorescence is detected in far red channels. This means that the dyes can be used in combination without significant overlap though it should be noted that in some circumstances, Nile Red can red-shift and fluoresce in the same channel as Nile Blue. Both Nile Blue and Nile Red are cell permeable, unlike other lipophilic probes such Oil Red O which requires that cells must be fixed prior to use. This provides an advantage as the Nile dyes can be used for live cell imaging and flow cytometry compared to classical lipophilic stains. To the best of our knowledge, neither are highly cytotoxic and pilot studies culturing cells with both dyes over several days did not result in significant cell death as observed with live cell fluorescent imaging.
What lipids do these dyes fluoresce in the presence of?
We tested over 75 different lipids with Nile Blue or Nile Red to assess by spectrofluorimetry which induce fluorescence of these probes. The following lists are based on the lipids we have tested. Others may be reported in the literature.
Nile Blue: Nile Blue fluoresces in the presence of unsaturated free fatty acids that are at least 16-carbons in length.
Lipids that induce Nile Blue to fluoresce include;
-Oleate.
-Nervonic acid (found in the brain and precursors to cerebrosides)
-Erucic acid (found in mustard oil and rapeseed oil but low in canola)
-Elaidic acid (found in low amounts in cow’s milk)
-Linoelaidic acid (an oil found in durian seeds)
-Palmitoleic acid (an omega-7 lipid)
-Arachidonic acid (a well-known polyunsaturated omega-6 fatty acid important in cellular signalling and eicanosoid biosynthesis).
Typically, the cis-versions of these lipids induced greater fluorescence compared to their trans-counterparts. Nile Blue shows remarkably tight specificity to these lipids (cis, >16-carbons, unsaturated free fatty acids).
Nile Red: Nile Red fluoresces in the presence of a broad spectrum of lipids that typically include;
-Triglycerides
-Cholesterol and cholesteryl esters
-some phospholipids
-some brain lipids.
Only one of the lipids that induce Nile Blue fluorescence also induce Nile Red fluorescence, oleate.
How to use these dyes for microscopy
As we aimed to use these dyes on sections, we assessed techniques for staining cryosections of unfixed tissues.
Protocol for section staining:
- Cryosection unfixed tissues: Temperatures for sectioning were adjusted depending on the tissue type. For instance, adipose tissue required the cryostat set to -30°C (the lowest temperature available on this instrument) whereas tissues such as the heart were sectioned at -15°C.
- Air-dry for 15 mins maximum: Typically, sections are left at room temperature to air-dry before stored at cooler temperatures. However, what we found was that lipids begin to deform after extended periods of drying, causing a destruction in cellular integrity and morphology.
- Fix tissues with 4% paraformaldehyde (in PBS) for 10-15 minutes. Avoid fixatives that remove lipids such as ethanol, methanol and acetone.
- Wash with PBS and stain with Nile Red (300 nM) and Nile Blue (5 µM) for 10 minutes, washed and mount with VectaShield containing DAPI.
- Imaging was typically performed on the same day, however, cellular integrity remained intact days after staining so long as stained sections were kept in the 4°C fridge.
What to expect to image with these dyes
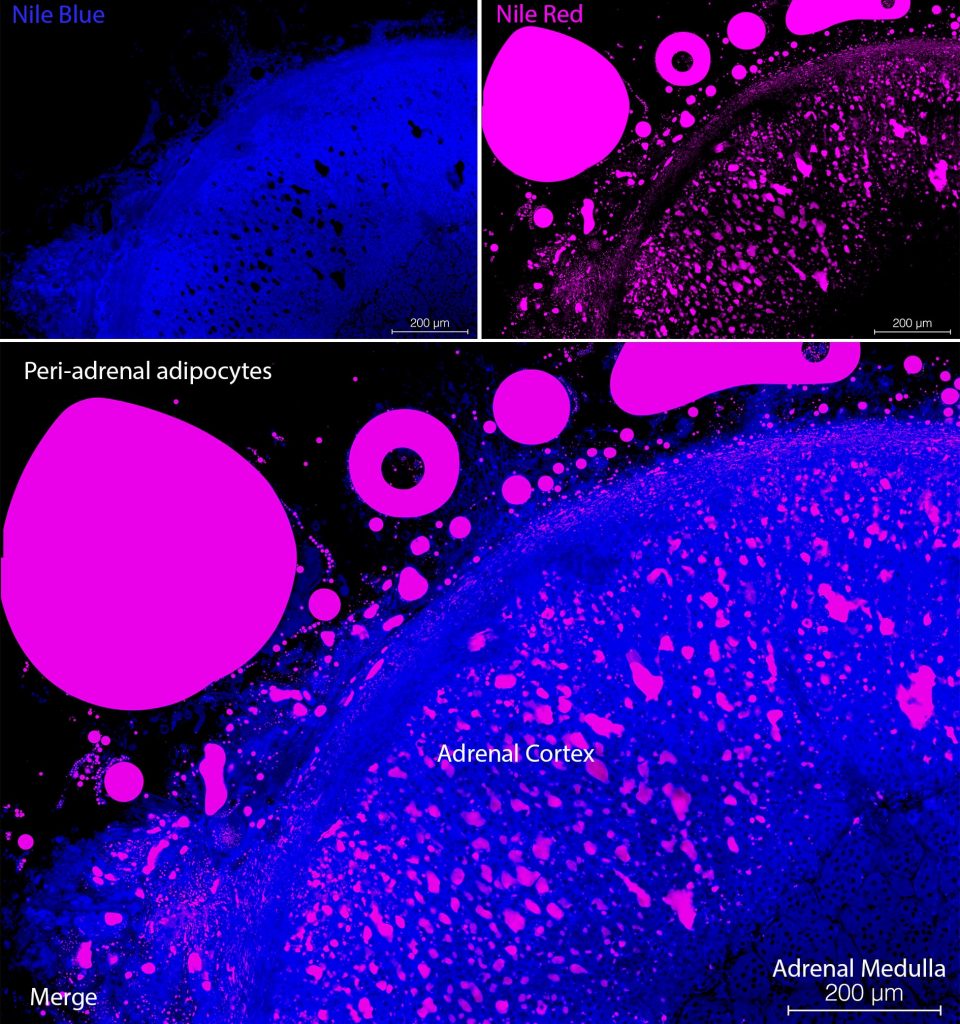
The typical Nile Blue-staining pattern for unsaturated free fatty acid in mammalian cells is a diffuse cytoplasmic stain (see Figure 1, top left panel). However, in the case of brown adipose cells, Nile Blue staining was observed in intense punctate structures, which might be peroxisomes. These structures had a core point of Nile Blue-staining surrounded with Nile Red signal. Nile Red typically stained droplets within cells (see Figure 2, top right panel). While some phospholipids induced fluorescence in spectrofluorimetry studies, cell membrane staining was not observed. Nile Red is seen in the orange-red range on confocal microscopes and Nile Blue signal can be seen in the far-red range. In tissues with cells containing droplets of retinol/Vitamin A, such as the hepatic stellate cells, also known as Ito cells, an autofluorescent signal can be detected in the violet channel. Do not use DAPI if you also wish to detect Retinol/Vitamin A in droplets in hepatic stellate cells as the fluorescent emission of DAPI and Vitamin A overlap. We recommend a counter stain which emits in the green range of the spectrum such as YOYO-1 for nuclei or Phalloidin-Atto488 for the cytoskeleton to identify individual cells. Nile Blue and Nile Red are cheap and effective fluorescent indicators of different lipids in mammalian cells which can be used simultaneously (see Figure 1, bottom panel). They can be combined with other autofluorescent signals such as Vitamin A, or fluorescently labelled antibodies. Due to their cell permeability, Nile Blue, Nile Red and other autofluorescent compounds can be used for flow cytometry.
The Authors
The authors are more than happy to help advise investigators with the use of these dyes for assessing lipid content inside cells.
Badwi Bob Boumelhem: badwi.boumelhem@sydney.edu.au
Stuart Fraser*: stuart.fraser@sydney.edu.au
School of Biomedical Engineering, Faculty of Engineering,
University of Sydney Camperdown, NSW, 2050, Australia
* Current address:
Molecular Cardiology, Centenary Institute,
Royal Prince Alfred Hospital, NSW, 2050, Australia


 (5 votes, average: 1.00 out of 1)
(5 votes, average: 1.00 out of 1)
Respected authors,
I am struggling to get the information on whether i can see the nile red staining in the paraformaldehyde fixed liver tissue sections obtained from mouse.
If yes, i processed them by clearing xylene, and grading them with alc, before i stained with nile red and observed them under immunoflourescence microscopy.
I cannot see any success in this..am I on right track.
Regards,
Dr. Pooja
Hi Dr Pooja,
Thanks for the question. My guess would be that the lipids recognised by Nile Red and Nile Blue have been dissolved and removed by the xylene and alcohol steps, greatly reducing the Nile Red fluorescent signal. Xylene and alcohol are good solvents of lipids. We don’t stain paraffin wax-embedded sections but rather cryostat sections with no exposure to xylene or alcohol.
Dear Stuart Fraser,
Is Nile Blue also showed fluorescence when excited with 488nm laser? I am getting the signal Nile blue signal in LD when excited by 488nm, apart from its far red emission via 633nm.
Hi
Thanks for sharing the protocol. Is it a good stain for liver steatosis study? Why is nucleus not visible?
Please inform.
thanks
Swati
Hi Swati,
Nile Red is an excellent stain for liver steatosis. It is easier to use than Oil Red O and cheaper than BODIPY.
Nile Red fluoresces in the presence of neutral lipids such as cholesterol and triglycerides but not DNA. Hence there’s no stain in the nucleus.
Hope this helps.
Hi Stuart,
Great article thanks. I’m interested in using Nile Red to stain for LD in the brain in frozen tissue. Do you think that it will work? I’m concerned about lipofuscin staining also.
Thanks,
Hannah