Technology Highlights – Expansion Microscopy
Posted by Marianna Poli, on 29 August 2023
Interview with Ana Agostinho and Steven Edwards, at the Advanced Light Microscopy facility at SciLifeLab in Sweden.
We are today talking about Expansion Microscopy imaging. Please provide a short summary of this type of imaging and list some applications:
Ana: My name is Ana Agostinho and I am a staff scientist at the Advanced Light Microscopy facility at SciLifeLab in Stockholm, Sweden, which is part of the Swedish National Microscopy Infrastructure, a Euro-BioImaging Node.
Steve: My name is Steven Edwards and I am working together with Ana at the Advanced Light Microscopy facility at SciLifeLab, Stockholm.
Ana: Our facility has been providing scientists with advanced sample preparation support for super-resolution microscopy techniques such as STED and SMLM. We have recently started offering scientists support with expansion microscopy, which enables super-resolution microscopy to be performed on diffraction limited microscopes. Without going into too much detail, the method involves crosslinking proteins in a fixed sample to a swellable polymer, which can be physically expanded by immersion in distilled water.
Using Expansion Microscopy in combination with confocal imaging, we have been able to clearly visualise the general architecture of mammalian centrioles (200 nm in diameter), as well as the fine details of the mitochondria inner membrane.
– Ana Agostinho, SciLifeLab
Steve: The technique is rapidly developing with many new articles and protocols published each year. We realised that this could be somewhat daunting for a biologist wishing to start with the method. We have chosen a single protocol, based on Magnified Analysis of the Proteome which is robust in our hands. It allows modest expansion (4-5x) of adherent cells.
Ana: We provide scientists with the reagents for gelation and digestion as well as access to simple laboratory equipment such as an oven and Eppendorf heater/shaker. We also give suggestions for sample mounting which must be stable enough to allow for long microscope acquisitions without drift.
Tell us a bit more about a specific project that was done in your facility using this technology? What scientific questions were you addressing?
Steve: Much of the work to-date has been focused on validating different expansion protocols and assessing their suitability for use in a facility environment. In order to provide adequate support, we have been focusing on the expansion of immunostained adherent cells.
Ana: With that being said, using this technique, in combination with confocal imaging, we have been able to clearly visualise the general architecture of mammalian centrioles (200 nm in diameter), as well as the fine details of the mitochondria inner membrane, typically imaged with super-resolution techniques (e.g. STED) (Figure, top panel and lower left panel). In addition, we have tested the possibility of further increasing the resolution when imaging our gels by combining expansion microscopy with super-resolution imaging modalities such as SIM and lattice SIM. This has enabled us to visualise the 9 microtubule triplets that form the centriole and fine details of the axoneme, in mammalian spermatocytes (Figure, lower right panel).
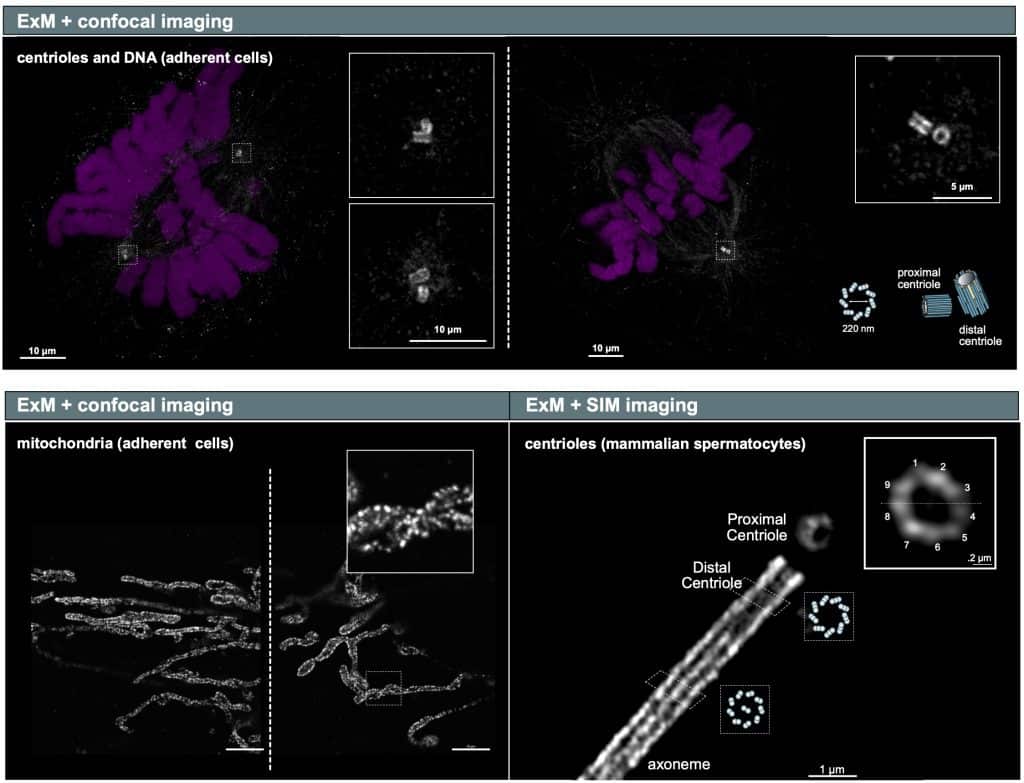
Example of Expansion Microscopy compared to other approaches (see text above for explanation). Image courtesy of Ana Agostinho, SciLifeLab, Swedish NMI.
What are some advantages of this technique that make it suited to addressing these types of questions?
Ana: Expansion microscopy is versatile with regards to the antibodies you can use to detect proteins of interest. In this case, we could visualise sub-cellular structures using the same primary antibodies we had already established in the lab, achieving sub 200 nm resolution on a confocal microscope.
Steve: The advantages of expanding samples are not limited to the spatial resolution improvement. Staining may also be improved as proteins are de-crowded and additional epitopes are made available.
What other services do you provide in your facility that would be useful in combination with this type of imaging?
Steve: We are fortunate to have a wide array of microscopes available to image expanded samples. Sub-50 nm resolution can be achieved with a simple point scanning confocal, but the airyscan detector from Zeiss is particularly suitable for detecting the weak signal from expanded samples. Successful application of this technique requires that the samples are treated with the same “care” that is required for other super-resolution modalites. We can therefore provide scientists with advice on fixation and labelling as part of the service.
Want to use Expansion Microscopy via the Euro-BioImaging service?
Euro-BioImaging is the European landmark research infrastructure for biological and biomedical imaging as recognised by the European Strategy Forum on Research Infrastructures (ESFRI). All scientists, regardless of their affiliation, area of expertise or field of activity can benefit from Euro-BioImaging’s pan-European open access services. By facilitating user access to high quality imaging facilities, resources, and services, with a constantly evolving technology offer, Euro-BioImaging will boost the productivity and impact of research across Europe.
Potential users of the Euro-BioImaging technologies are encouraged to submit project proposals via our website. To do so, you can LogIn to access our application platform, choose the technology you want to use and the facility you wish to visit, then submit your proposal. By using Euro-BioImaging, users will benefit from advice and guidance by technical experts working at the Nodes, training opportunities, and data management services.
For more information visit our website at www.eurobioimaging.eu or contact us at info@eurobioimaging.eu


 (1 votes, average: 1.00 out of 1)
(1 votes, average: 1.00 out of 1)