Illuminating the Brain: Microscopy Techniques in Neuroscience and My Applications in the Lab
Posted by Sally Horton, on 23 June 2025
Understanding the brain’s complexity requires more than theory or behaviour; it demands a look at the smallest units of structure and function. Microscopy has long been a cornerstone of neuroscience, evolving rapidly from conventional optical methods to cutting-edge super-resolution and electron techniques. This article explores how confocal microscopy, STORM, and electron microscopy are advancing our understanding of neural circuits, with examples of how these techniques furthered my PhD research.
Confocal Microscopy: A Foundation for Neural Imaging
Confocal microscopy is a powerful imaging technique widely used in neuroscience for visualising fluorescently labelled neurons in either two or three dimensions. Unlike widefield microscopy, which collects light throughout the depth of a specimen, confocal microscopy enhances resolution and contrast by using point illumination and a spatial pinhole to eliminate out-of-focus light1,2. This makes it well-suited for imaging brain tissue sections. Key advantages include improved optical resolution and contrast, reduced background noise, and clearer imaging in dense tissue1–7. Confocal microscopy enables optical sectioning and 3D reconstruction of complex structures such as dendrites, axons, and organelles. It supports fluorescent multiplexing, allowing simultaneous imaging of multiple labelled targets to study molecular interactions1–7. Its capacity for live-cell imaging makes it valuable for tracking dynamic processes, such as neurite outgrowth or dendritic dynamics8. Additionally, it facilitates quantitative analysis of fluorescence intensity, molecular distribution, and spatial organisation. Overall, confocal microscopy is a foundational tool in neuroscience for investigating cellular architecture, monitoring synaptic changes, and mapping neural circuits with high clarity and precision1–8.
Common uses of confocal microscopy:
- Imaging the morphology and structure of neurons (dendrites, axons, somata) and glial cells (astrocytes, microglia, oligodendrocytes)
- Analysing dendritic spines (density, shape, and dynamics) and synaptic structures
- Assessing protein localisation and colocalisation by examining their spatial relationships using multi-channel fluorescence
- Mapping neural circuits using fluorescent tracers or genetically encoded reporters to study network architecture
- Live-cell imaging of dynamic processes by monitoring real-time events such as vesicle trafficking or neurite outgrowth in live cells
- Quantitative fluorescence analysis by measuring expression levels, signal intensity, and spatial distribution of markers with high precision
Throughout my PhD, I used confocal microscopy to map the spatial distribution of excitatory and inhibitory synapses along individual dendritic branches of hippocampal neurons, either dissociated or in slices of tissue (Figure 1). Such neurons were imaged using confocal microscopy at different developmental timepoints, enabling me to map synaptic distribution at a relatively high spatial resolution, and track any changes over time.
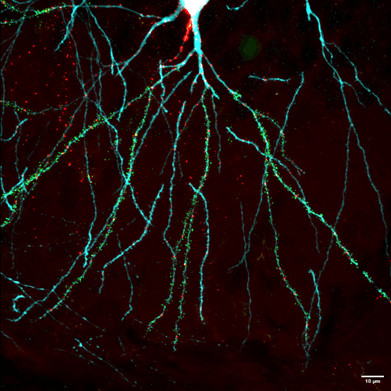
Figure 1: Example image of a pyramidal neuron (postnatal day 21) acquired using confocal microscopy. Neuronal structure is outlined (cyan), along with inhibitory synapses (red), and excitatory synapses (green). Image sourced from Horton et al, 20249.
STORM and Super-Resolution: Breaking the resolution limit
Super-resolution imaging techniques overcome the diffraction limit of conventional light microscopy, achieving lateral resolutions of approximately 20-30nm, an order of magnitude beyond standard confocal systems 10–14. This increase in resolution is highly beneficial for neuroscience research, where many biologically significant structures, such as synaptic vesicles, cytoskeletal elements, and ion channels, exist at the nanoscale and cannot be resolved with traditional optical approaches. One super-resolution imaging approach that I used throughout my PhD is direct Stochastic Optical Reconstruction Microscopy (dSTORM). dSTORM operates by stochastically activating and localising individual fluorescent molecules in a sample across many imaging cycles, then reconstructing a high-resolution image from the precise coordinates of these single-molecule events 13–16. This enables researchers to map the spatial distribution of proteins with precision, revealing patterns of organisation, clustering, and colocalisation. Elucidating such features can be crucial for understanding synaptic function, signal transduction, and membrane dynamics.
In neuroscience, STORM has become a transformative tool for:
- Visualising postsynaptic density (PSD) architecture and nanodomains
- Investigating receptor distributions at individual synapses
- Studying synaptic vesicle organisation in presynaptic terminals
- Analysing nanoscale changes in protein localisation in models of neurodegeneration or plasticity
Throughout my PhD, I used dSTORM to investigate the organisation of protein clusters in inhibitory synapses, and how these changed throughout development (Figure 2). Specifically, we used dSTORM to assess any potential changes in volume of VGAT puncta throughout development. Such imaging, which was undertaken in fixed brain slices, revealed that VGAT puncta do not change in volume throughout development (Figure 2). However, although VGAT puncta volume remained unchanged, confocal imaging revealed that inhibitory synapses serve as a structural scaffold during development, prior to the formation of a fully developed active zone9.
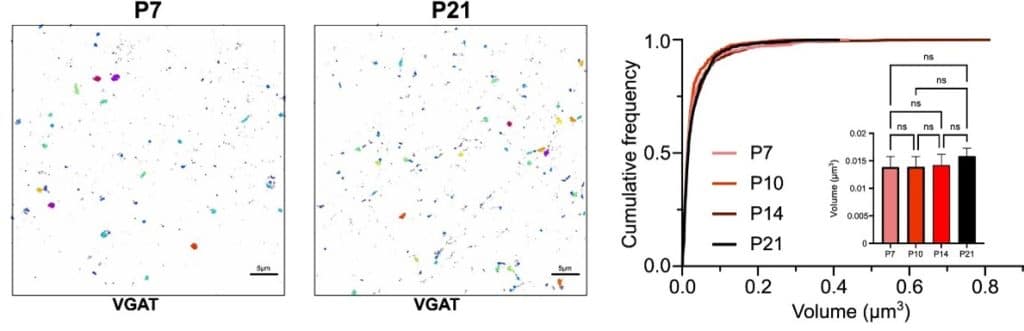
Figure 2: Example dSTORM images of VGAT puncta in fixed brain slices at both postnatal day 7 (P7) and 21(P21). Cumulative volume distributions of VGAT clusters across developmental stages (P7, P10, P14, and P21) revealed no significant difference. Image sourced from Horton et al, 20249.
Electron Microscopy: The Gold Standard for Ultrastructure
Electron microscopy (EM) offers an unmatched level of spatial resolution, making it an indispensable tool for studying the ultrastructure of the nervous system. Unlike light microscopy, EM can resolve features at the nanoscale, enabling the visualisation of, for example, individual synapses, intracellular organelles, and cytoskeletal elements with extraordinary clarity. In neuroscience, EM techniques such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are used to reconstruct neural circuits in exquisite detail, in order to understand the intricate architecture of the brain17–20. TEM provides high-resolution, two-dimensional images by passing electrons through ultrathin tissue sections, revealing fine subcellular detail crucial for identifying, for example, synaptic vesicles, membrane specialisations, and mitochondrial morphology. In contrast, SEM, particularly in its advanced form as serial block face-SEM (SBF-SEM), enables three-dimensional reconstruction of tissue volumes. This is particularly powerful for connectomics, enabling us to understand the wiring dynamics of neural circuits, by capturing many serial images to reconstruct the spatial relationships between neurons and their synaptic connections 9,21. Together, EM techniques allow researchers to probe the structural basis of brain function and dysfunction at an unprecedented scale, contributing to our understanding of development, plasticity, and disease mechanisms. As neuroscience moves toward increasingly integrative and multiscale models of brain organization, EM continues to serve as a critical bridge between molecular detail and systems-level insight.
EM techniques are key for:
- Mapping connectomes with nanoscale precision, by identifying synaptic connections between individual neurons
- Analysing mitochondrial morphology in neurodegeneration
- Studying myelination and axonal integrity to visualise the ultrastructure of myelin sheaths and detect demyelination or axonal injury
- Identifying synaptic vesicle distribution and docking to understand presynaptic function and neurotransmitter release dynamics
- Assessing organelle contact sites to study inter-organelle communication
- Studying microglial and astrocytic morphology during neuroinflammation
- Monitoring neurodevelopmental ultrastructure to examine growth cone morphology, synaptogenesis, and axonal pathfinding
- Analysing synaptic plasticity mechanisms to observe structural changes in dendritic spines
During my PhD, I specifically used SBF-SEM to examine the changes in synapse formation and features throughout development, and to examine the spatial distribution of excitatory and inhibitory synapses along individual dendritic branches with high resolution (Figure 3). This revealed that there is a tight subcellular balance between excitation and inhibition.
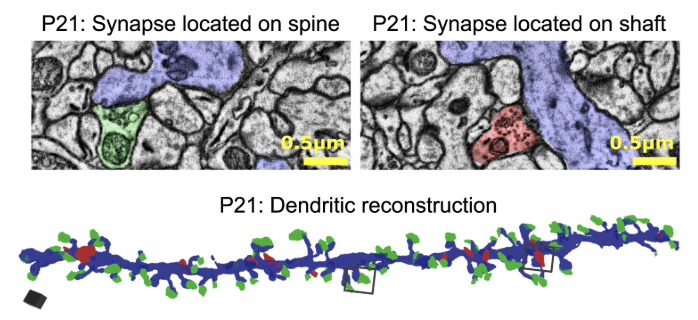
Figure 3: Example single-plane images acquired using SBF-SEM of synapses located on a dendritic spine (left, excitatory) and on the dendritic shaft (right, inhibitory). Below shows a three-dimensional reconstruction of a dendritic branch showing all impinging synapses (excitatory are shown in green, and inhibitory in red). Image sourced from Horton et al, 20249.
Conclusion: A Microscopic Window into the Brain
Each of these techniques, confocal, dSTORM, and EM, offers unique advantages. Used together, they provide a comprehensive picture of neural structure at different spatial scales and can offer insights into function. As tools improve and workflows integrate (e.g., correlative light and electron microscopy), the future of brain imaging looks ever more precise and promising. Throughout my doctoral research, the integration of multiple imaging modalities allowed me to map the spatial distribution of excitatory and inhibitory synapses, to reveal a tight matching between excitation and inhibition, which sharply declined throughout development1. These findings not only contributed to a deeper understanding of neurodevelopment but also demonstrated how complementary imaging approaches can enrich neurobiological discovery.
Bibliography
1. Conchello, J.-A. & Lichtman, J. W. Optical sectioning microscopy. Nat Methods 2, 920–931 (2005).
2. Elliott, A. D. Confocal Microscopy: Principles and Modern Practices. Curr Protoc Cytom 92, e68 (2020).
3. Webb, R. H. Confocal optical microscopy. Reports on Progress in Physics 59, 427–471 (1996).
4. Smith, C. L. Basic Confocal Microscopy. Curr Protoc Mol Biol 81, (2008).
5. Paddock, S. Over the Rainbow: 25 Years of Confocal Imaging. Biotechniques 44, 643–648 (2008).
6. Cox, G. Biological confocal microscopy. Materials Today 5, 34–41 (2002).
7. Amos, W. B. & White, J. G. How the Confocal Laser Scanning Microscope entered Biological Research. Biol Cell 95, 335–342 (2003).
8. Zaccard, C. R., Kirchenbuechler, D., Yoon, S., Arvanitis, C. & Penzes, P. Protocol for live enhanced resolution confocal imaging of dendritic spinule dynamics in primary mouse cortical neuron culture. STAR Protoc 2, 100427 (2021).
9. Horton, S. et al. Excitatory and inhibitory synapses show a tight subcellular correlation that weakens over development. Cell Rep 43, 114361 (2024).
10. Galbraith, C. G. & Galbraith, J. A. Super-resolution microscopy at a glance. J Cell Sci 124, 1607–11 (2011).
11. Xu, J., Ma, H. & Liu, Y. Stochastic Optical Reconstruction Microscopy (STORM). Curr Protoc Cytom 81, 12.46.1-12.46.27 (2017).
12. Betzig, E. et al. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science (1979) 313, 1642–1645 (2006).
13. Heilemann, M. et al. Subdiffraction‐Resolution Fluorescence Imaging with Conventional Fluorescent Probes. Angewandte Chemie International Edition 47, 6172–6176 (2008).
14. Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods 3, 793–796 (2006).
15. Endesfelder, U. & Heilemann, M. Direct Stochastic Optical Reconstruction Microscopy (dSTORM). in 263–276 (2015). doi:10.1007/978-1-4939-2080-8_14.
16. van de Linde, S. et al. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat Protoc 6, 991–1009 (2011).
17. Anderson, J. C., Douglas, R. J., Martin, K. A. C. & Nelson, J. C. Map of the synapses formed with the dendrites of spiny stellate neurons of cat visual cortex. Journal of Comparative Neurology 341, 25–38 (1994).
18. GRAY, E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat 93, 420–33 (1959).
19. White, E. L. & Rock, M. P. Three-dimensional aspects and synaptic relationships of a Golgi-impregnated spiny stellate cell reconstructed from serial thin sections. J Neurocytol 9, 615–636 (1980).
20. Denk, W. & Horstmann, H. Serial Block-Face Scanning Electron Microscopy to Reconstruct Three-Dimensional Tissue Nanostructure. PLoS Biol 2, e329 (2004).
21. Rigby, M. et al. Multi-synaptic boutons are a feature of CA1 hippocampal connections in the stratum oriens. Cell Rep 42, 112397 (2023).


 (No Ratings Yet)
(No Ratings Yet)
