Featured image with Saurabh Chand Sagar
Posted by FocalPlane, on 25 April 2025
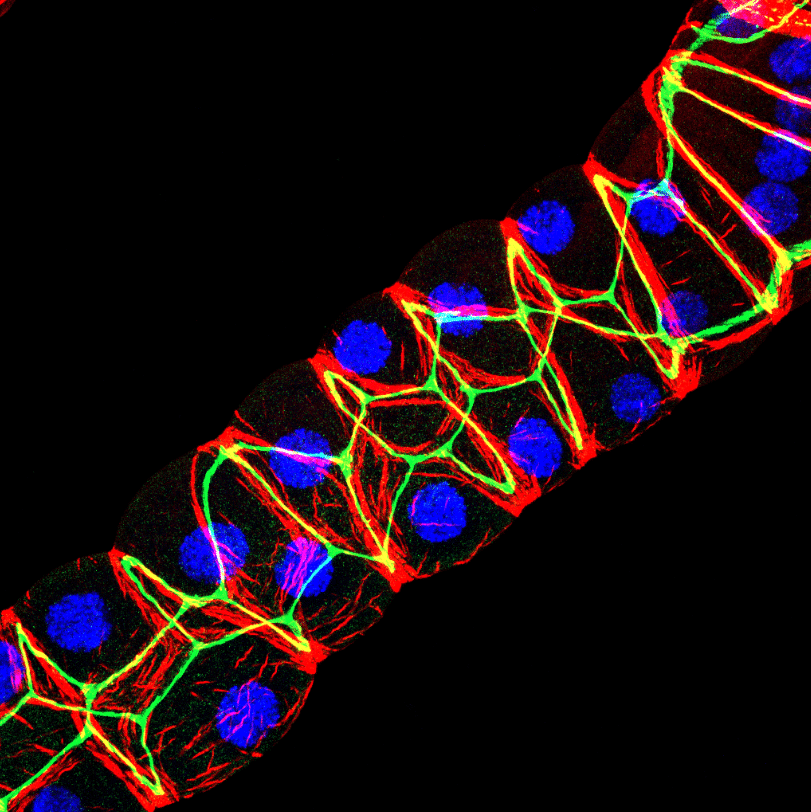
Our featured image, acquired by Saurabh Chand Sagar, presents a laser scanning confocal projection of the Malpighian tubules (MTs) from third instar wandering-stage (118 ± 2 hours post-eclosion) wild-type Drosophila melanogaster larvae. In the image, red denotes F-actin protein, green highlights Discs Large, a protein involved in maintenance of apicobasal polarity, and blue marks the nuclei within the MTs. The MTs were carefully dissected, fixed in 4% paraformaldehyde, and subjected to immunostaining using anti-mouse primary antibody against dlg1 (DHSB, 4f3), Phalloidin AF 550 for F-actin, and DAPI for nuclear labelling. Imaging was performed using a Carl Zeiss LSM 900 META confocal microscope, and the resulting images were analysed with ImageJ software. A maximum intensity 3D projection of the data was generated for visualisation.
Discover more about Saurabh’s research
Research career so far: I am a Drosophila cell biologist at the Department of Zoology, Institute of Science, Banaras Hindu University (BHU), where I have been pursuing my research since 2021. I hold both a Bachelor’s and Master’s degree in Zoology from BHU. In addition, I have been awarded the prestigious CSIR-JRF fellowship (AIR 114) and qualified for the GATE examination in my first attempt. My research expertise includes Drosophila genetics, GAL4-UAS-based genetic manipulation, dietary regulation for drug testing, and dissection of tissues in both larval and adult stages. I am also proficient in handling mice and rats for in vivo experiments, including surgical procedures and drug administration. My technical skills cover a wide range of methodologies, such as immunostaining, western blotting, immunoprecipitation, PCR, qRT-PCR, spectrophotometry, as well as confocal, DIC, and polarizing microscopy. Additionally, I am experienced in biomolecule extraction and conducting behavioural assays, including longevity, fecundity, and developmental assays. I am skilled in using various data analysis tools, including GraphPad Prism, ZEN, ImageJ, and Adobe Photoshop. Outside of my research, I enjoy puzzle solving, painting, sketching, and listening to music. To date, I have published seven book chapters and communicated one paper, which is currently under revision.
Current research: Currently, I’m working as PhD scholar under the supervision of Prof. Madhu G. Tapadia at Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi, India, 221005. My research focusses on elucidating the molecular mechanisms underlying tissue morphogenesis, with a particular emphasis on the roles of caspase-3 and RhoGTPase in F-actin-associated morphogenesis within the Malpighian tubules of Drosophila Drice mutants. Additionally, I am investigating the effects of Ayurvedic Rasayana on tissue morphogenesis within the same genetic background.
Favourite imaging technique/microscope: My favourite imaging techniques include confocal laser microscopy and DIC microscopy. I like confocal because of the detailing it provides once the target protein/organelle is stained with some fluorochrome. However, a confocal microscope has the limitation to fix and stain the tissue/organs before visualization under the microscope which can be overcome by using DIC microscopy. DIC provides a pseudo-3D image of the unstained specimen, which makes it more suitable to look at the various organelles within the cells/tissues. My favourite microscope is Carl Zeiss LSM 900, this is a user-friendly upright laser based confocal microscope nearly perfect to visualise any prepared slide stained with fluorochrome/fluorescent molecules.
What are you most excited about microscopy? To be able to visualize the tiniest details within the cells using the microscopes. Specially, the recent advances in electron microscopy makes it possible to look closely within the cells and tissues.


 (60 votes, average: 1.00 out of 1)
(60 votes, average: 1.00 out of 1)
I am voting for picture no 5.
Wonderful image
I am voting for image 5.
Image 5. Captivating high resolution image.
Am voting for pic no 5
I am voting for picture no 5.
Very impressive
I am voting picture no 5
Very nice 👍
Thaink
Beautifully captured! I vote for Image no. 5
I am voting for Sourav Chand Sagar picture no. 5.