LSFM series – Surfing on the data freak wave! PART I: Knowing your turf, knowing your surf!
Posted by Elisabeth Kugler, on 5 September 2020
Elisabeth Kugler 1-3 and Emmanuel G. Reynaud 4
Contact: kugler.elisabeth@gmail.com; emmanuel.reynaud@ucd.ie
For the last two decades, advanced image acquisition has become an essential part of the biological sciences, even if it means that this came with the guilty pleasure of using Photoshop to misrepresent imaging data. Things have evolved drastically, with 3D imaging technologies having moved far beyond single 2D images, offering up new opportunities but; but there is a catch! Moving from one dimension to two, three, four, or more means more data. Especially in Light Sheet Fluorescence Microscopy (LSFM) this creates a flood of large stacks building into a freak wave of data, swamping many researchers and facility managers alike. Here we present a series of five blog posts with tips and tricks to help you regain control and surf with total confidence the data freak wave!
LSFM blog programme:
- The basics of LSFM (September 2020)
- Improving sample mounting (October 2020)
- Calibration and Acquisition (November 2020)
- Tailoring the data (December 2020)
- What is next? AI and smarter than us LSFM (January 2021)
First things first, are we all talking about the same thing?
There is a difference between talking about an imaging technique and knowing how to dismantle and reassemble a microscope. Light Sheet Microscopy is one of the most diverse imaging techniques but one thing all setups have in common is the use of a sheet of light to optically section the sample. With more than 128 acronyms so far (Fig. 1), this illumination approach is used in a large variety of light microscopy setups, including super-resolution, mesoscopy, and cytometry.
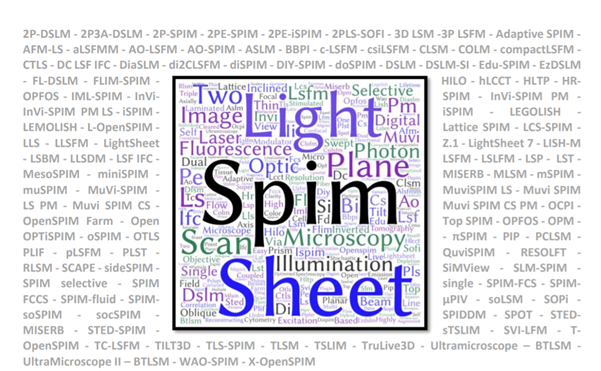
Figure 1 – The Light Sheet Acronym Overload Disorder – a word cloud of the LSFM associated acronyms illustrating the overuse of SPIM, Sheet/Plane, and Illumination alongside 128 acronyms (grey) recorded so far.
Light sheets can be created by different means and are generally used for two distinct reasons. Firstly, for the last three decades, light sheets have been used to define a line that specifically scans surfaces, also known as Line Scanners. Those are used to scan things such as cathedrals, mechanical parts, tree trunks, and most famously bar codes in nearly every supermarket worldwide (Fig. 2A). In this case, the line creates an inverse pattern as the black lines of the bar code absorb the red light, which is captured by the camera that processes it, and provides the bar code specific print to the cashier software. Line scanners are used to scan the surfaces of object by sweeping the light sheet (Fig. 2B). This principle can be applied in light sheet microscopy when your sample is not fully transparent or contains parts that may block the light sheet, allowing you to scan its surface. Therefore, every Light Sheet Microscope is a potential micro Line Scanner that can scan tiny structures such as insect heads (Fig. 2E). Secondly, in a Light Sheet Microscope, the light sheet is used to define a plane in space, which is only effective if the light sheet illuminates the focal plane of the detection objective as this coupling is essential to combine the illumination and the detection orthogonally. This means that the light sheet is fixed and, thus, the sample is moved through the light sheet, which also generates various mechanical and sample drift issues. However, similarly in Particle Image Velocimetry (PIV, Fig. 2C), the sample can be moved through a light sheet by flow of a gaseous or liquid medium. In PIV this allows the user to track particles, while in light sheet microscopy this can be applied to fluorescence cross-correlation spectroscopy (FCCS) or flow cytometry of organoids.
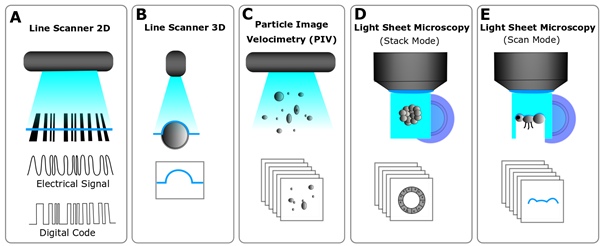
Figure 2- Uses of light sheets – A. A bar code reader use a light sheet to create a line to read a code. B. A line scanner with a camera at a narrow angle (45°) use a line to register the contours of an object. C. Particle Image Velocimetry (PIV) is used in flow visualization and uses a light sheet to optically section a plane and trace particles. D. A LSFM uses a light sheet to optically section a translucent/transparent object creating an illuminated plane with a camera at 90°, E. It can also “read” the surface of an object and behave like a line scanner.
So, what LSFM are you talking about?
LSFMs can be set up with a variety of geometry (Fig. 3). The well-known SPIM (Selective Plane Illumination Microscope) is a horizontal set up generating a vertical light sheet (Fig. 3B), while the Ultramicroscope is an upright system generating a horizontal light sheet (Fig. 3A). You can also invert the entire microscope, tilt it (Fig. 3C), or use a single objective instead of the canonical two (Fig. 3D). Finally, the light sheet can be generated in the microscope by using a mirror, which can be either mounted on an objective or inserted in a specific microdevice (e.g. prism or plate; Fig. 3E).
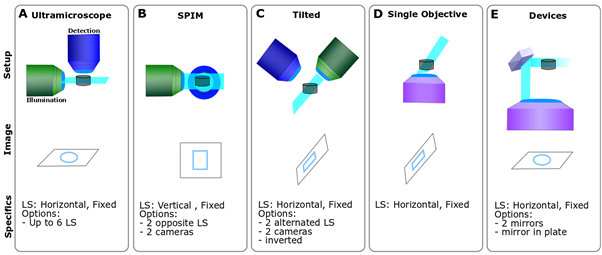
Figure 3- LSFM geometrical diversity – A. A Ultramicroscope is an upright system and the sample can be flat on a surface. B. SPIM is a horizontal set up and the sample is either held form above or underneath C. A tilted LSFM can use a single objective for scanning or alternate between both. the sample is usually flat on a surface with the light sheet being skewed at an angle. D. Single objective systems require very specific objectives to allow the generation of the light sheet while scanning and generate a skewed light sheet. E. Devices: those systems require a reflective element either a mirror or a prism to achieve the optimal configuration.
This first blog exemplifies that lights sheet microscopes are an optical specie that is part of a very large family. Not only can the microscope geometry be very different, but it can also be used to scan surfaces, generate stacks, or detect object in a flow. It can also alternate illumination between opposing objectives. However, the way the light sheet itself is generated can be drastically different. From a fixed Gaussian beam using a single cylindrical lens to a more advanced combination of Bessel beams, Structured illumination, and galvanometers in a Lattice Light Sheet Microscope.
The take home message is that there are as many light sheets as light sheet microscopes, e.g. fixed, scanned, tilted, various thicknesses, field of views, or even sweeping light sheets, which can scan larger areas by displacing the optimal waist across the field of view. All of these characteristics have important consequences for image acquisition and so the final data load. In the following blog posts, we will discuss what can be done to improve your data flow in LSFM.
May the LISH be with you,
Elisabeth and Emmanuel
P.S.: If you want to share your views, do not hesitate to get in touch.


Associations / Institutes
(1) Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Medical School, Beech Hill Road, Sheffield, S10 2RX United Kingdom. (2) The Bateson Centre, Firth Court, University of Sheffield, Western Bank, Sheffield, S10 2TN United Kingdom. (3) Insigneo Institute for in silico Medicine, The Pam Liversidge Building, Sheffield, S1 3J United Kingdom. (4) School of Biomolecular and Biomedical Science, University College Dublin, Belfield, Dublin 4. Ireland.
Acknowledgements
We would like to thank our free of charge reviewers Kate McDole, Agnese Kocere, and Tim Chico for their incredibly helpful and witty comments.
Further Reading:
- Reynaud, E.G., Peychl, J., Huisken, J. and Tomancak, P. (2015). Guide to light-sheet microscopy for adventurous biologists. Nature Methods 12: 30–34
- Olarte, O.E., Andilla, J., Gualda, E.J. and Loza-Alvarez, P. (2018) Light-sheet microscopy: a tutorial. Adv. Opt. Photon. 10: 111-179
- Chang, B-J, Dean, K.M. and Fiolka, R. (2020) Systematic and quantitative comparison of lattice and Gaussian light-sheets. Opt. Express 28: 27052-27077


 (3 votes, average: 1.00 out of 1)
(3 votes, average: 1.00 out of 1)
Well written… good job.