Featured image with Theresa Wiesner
Posted by FocalPlane, on 21 June 2024
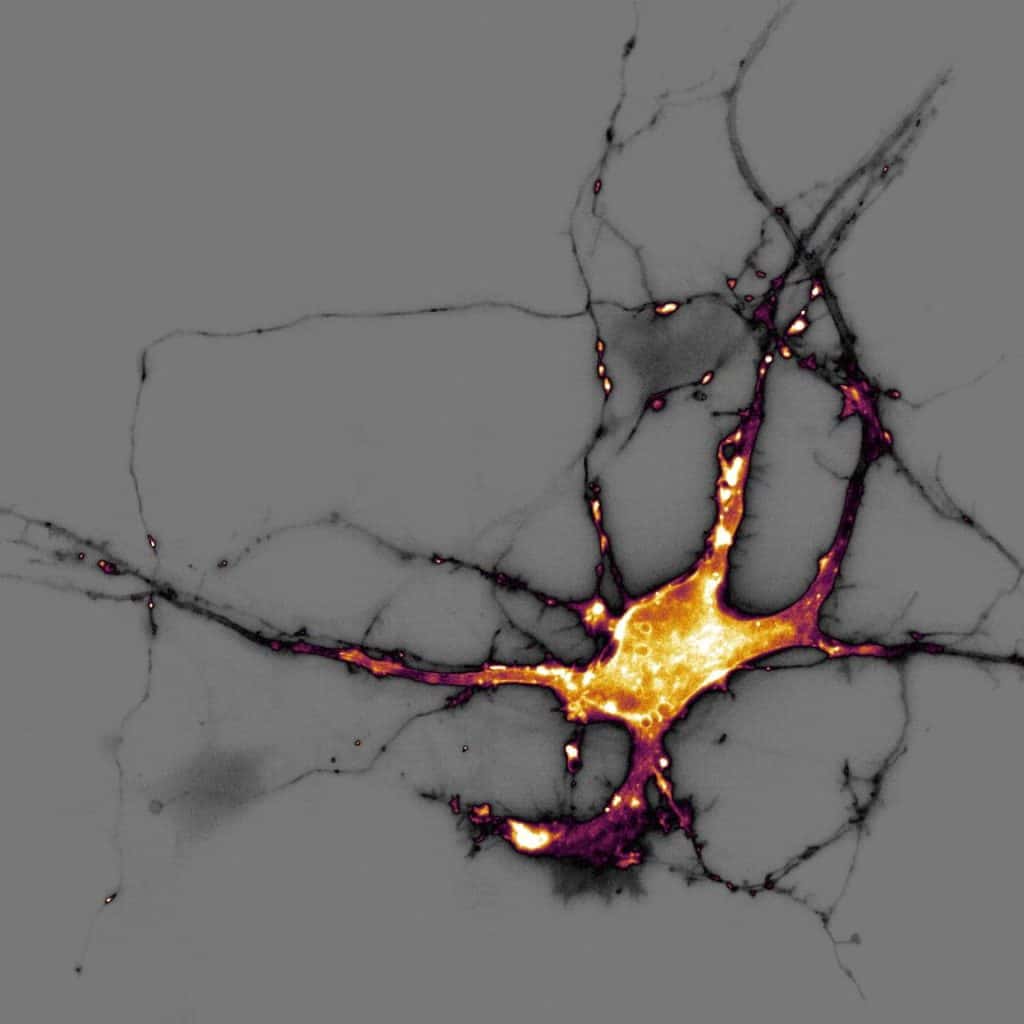
Our featured image, acquired by Theresa Wiesner, shows a neuron in dissociated hippocampal culture and was imaged using highly inclined thin illumination microscopy (HiLO). The neuron is transfected with a neurotransmitter (glutamate) sensor and shown in orange. The image was acquired using Nikon Elements software / microscope and post-processing was done using FIJI (ImageJ).
Find out more about Theresa’s research below:
Research career so far: Coming from cancer research with focus on high throughput screening for drug development in the early years of my bachelor at the University of Perugia (Italy), I got fascinated during an internship with functional assays such as field recordings to study neuronal cell behavior. I got hooked on doing my master in bio-photonics to learn functional imaging at Université Segalen in Bordeaux (France). During my Ph.D in Biophotonics at Université Laval, Quebec (Canada), I combined functional assays, super-resolution imaging and computational tools to quantitatively assess and explore synaptic protein organization and remodeling due to synaptic plasticity.
I am currently a postdoctoral fellow in the NeuroCyto team at the Institute of NeuroPhysiopathology (INP) in Marseille, France. Here I am studying the role of actin/spectrin submembrane scaffold in regulating axonal exocytosis by combining live-cell and super-resolution microscopy.
Spurred by my Ph.D. and postdoctoral experience, my ambition is to overcome the current inability to directly link nanoscale protein organization to neuronal function impedes our understanding of fundamental cellular mechanisms and our capacity to alleviate misorganization in neurological disorders.
Current research: Neurons form vast-ranging axons to establish the connections that ensure the flow of information throughout the brain. This polarized and complex arborization primarily depends on the axonal cytoskeleton, with defects leading to severe neurological dysfunction. A periodic scaffold recently discovered thanks to super-resolution microscopy lines the internal side of the plasma membrane along axons. The functions of this scaffold remain poorly known, and we propose that it regulates neuronal activity by acting as an insulating layer that prevents vesicles transported inside the axon to spuriously fuse with the plasma membrane along the shaft. It would thus restrict the fusion process (called exocytosis) to controlled places, such as presynaptic boutons along the axon that are devoid of it. I am employing fast live-cell imaging to detect neuronal activity along the axon and super-resolution microscopy to resolve the underlying periodic scaffold. Insights from this work will provide new understanding into the consequences of misorganization in neuropathologies.
Favourite imaging technique/ microscope: I love live-cell imaging of neuronal activity! It is like whale-watching or star-gazing: one never knows when and where something will happen. It is just astonishing the variety of ways neurons can be active! Combine that with the unprecedented resolving power of fluorescence, super-resolution microscopy of neuronal proteins and suddenly one can picture a new universe forming. A nanoscale world where neuronal protein organization dictates the precise neuronal activity within the neuronal compartments!
What are you most excited about in microscopy? I am most excited about developing multi-modal imaging techniques that allow for excellent spatial and temporal resolution. Correlative live-cell and super-resolution microscopy is one such technique that is very promising for understanding the impact the organization of protein assemblies has on cellular function. Additionally, I am also very excited about the increasing use of machine learning algorithms to help us extract all the hidden information from our images and help us in maximizing what we get from image acquisition.


 (2 votes, average: 1.00 out of 1)
(2 votes, average: 1.00 out of 1)