Featured image with Shivangi Verma
Posted by FocalPlane, on 30 August 2024
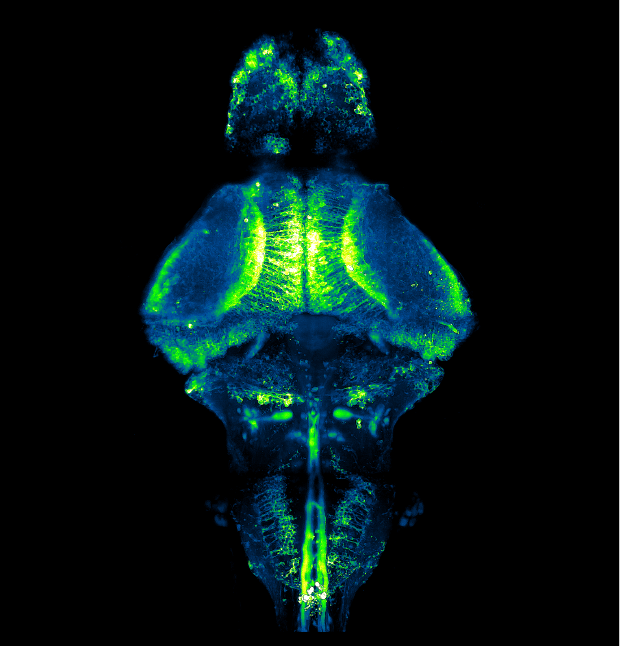
Our featured image, acquired by Shivangi Verma, gives us a peek into the brain of a zebrafish. It depicts neurons in the whole brain of a larva expressing GCaMP6f, a fluorescent reporter of neuronal activity, using a pan-neuronal promoter. A 10-day old transparent homozygous albino larva was embedded in agarose and imaged using ZEISS Lightsheet 7 microscope. The tiled images were first stitched using Zen Black and subsequently processed in Fiji to obtain a maximum-intensity projection.
Find out more about Shivangi’s research below:
Research career so far: At school, I was always captivated by biology and the technical advancements in the field of biological sciences. This encouraged me to go for an undergraduate degree in biomedical science. As a master’s student in Prof. Suman Dhar’s lab at SCMM (Delhi), I studied Helicobacter pylori, an opportunistic bacteria that inhabits about half of the human population worldwide and can cause gastric cancers. I was interested in the unique biochemical pathways that can be specifically targeted in this pathogen, sparing the resident gut microbiome. I developed an inclination for Neurobiology upon attending a talk from Prof. David Anderson, where he discussed his lab’s work on optogenetic control of aggression. I was intrigued by his findings and decided to apply for a PhD in Neurobiology. I went on to join NCBS where, under Prof. Vatsala Thirumalai’s guidance, I have investigated the implications of cerebellar synaptic refinement on motor circuit and behaviour of larval zebrafish. During my PhD, I participated in a Neuromatch summer course that equipped me with computational skills and enabled me to thoughtfully design experiments and analyse the electrophysiology and imaging data more effectively. My experience in the lab was also complemented by the Neural Systems & Behavior summer course at the Marine Biological Laboratory (Woods Hole). This is where I first explored various scales of neuroscience, from cellular to behavioural, by working with organisms like leeches, crabs, fruit flies and worms. I learned to appreciate the idea of choosing the right model system for every question. The rich ecosystem of Woods Hole has also sparked an interest to study the neuronal circuits in unconventional marine animals. Going forward, I want to study the development and function of nervous systems using unconventional animal models and attempt to understand the mysterious ways in which neural circuits have evolved.
Current research: As a graduate student at the National Centre for Biological Science (Bangalore), I have primarily studied the functional implication of synaptic pruning using larval zebrafish at the level of single neurons, circuit and behaviour. During early development, the redundant synapses in the brain are judiciously pruned, giving rise to a functional circuit. The sheer number of synapses makes it difficult for biologists to study direct functional implications of synaptic pruning. To overcome this challenge, I utilised a pair of unique neurons in the olivo-cerebellar network that exhibit a one-to-one mapping through pruning: the inferior olivary neurons and the cerebellar Purkinje neurons. I have investigated this temporally precise pruning using a combination of in vivo whole cell patch clamp electrophysiology with fictive motor recordings, calcium imaging and behaviour. Observations from this study provide insights into the processes underlying functional circuit formation.
Favourite imaging technique/microscope: Functional brain imaging with light-sheet microscopy has excited me the most. It is fascinating to see how scientists have complemented traditional electrophysiology with calcium and voltage sensors to monitor neuronal activity in animals during behaviour. Imaging speed is critical for capturing fast electrical signals using these sensors in 3-dimensional brain tissue, and light-sheet microscopy has been a game changer, making this high-speed imaging possible.
What are you most excited about in microscopy? I am excited about the incredible progress in functional brain imaging which has revolutionised neuroscience. Biological processes occur in a wide range of spatiotemporal scales. For example, transmission of electrical signals in the brain occurs in microseconds. Using electrophysiology, we can capture these electrical signals, however with poor spatial resolution. With the advent of fast imaging and development of sensors of neuronal activity, we are now able to observe a large number of neurons simultaneously. This extends to the whole brain in case of small animals like zebrafish, danionella, jellyfishes and worms. However, this comes at the cost of temporal precision. I look forward to the upcoming advancements in fast imaging techniques and sensors which will allow us to see through the cells in all kinds of 3-dimensional tissues with high spatiotemporal precision during natural behaviours.


 (2 votes, average: 1.00 out of 1)
(2 votes, average: 1.00 out of 1)