Featured image with Maik Bischoff
Posted by FocalPlane, on 18 July 2025
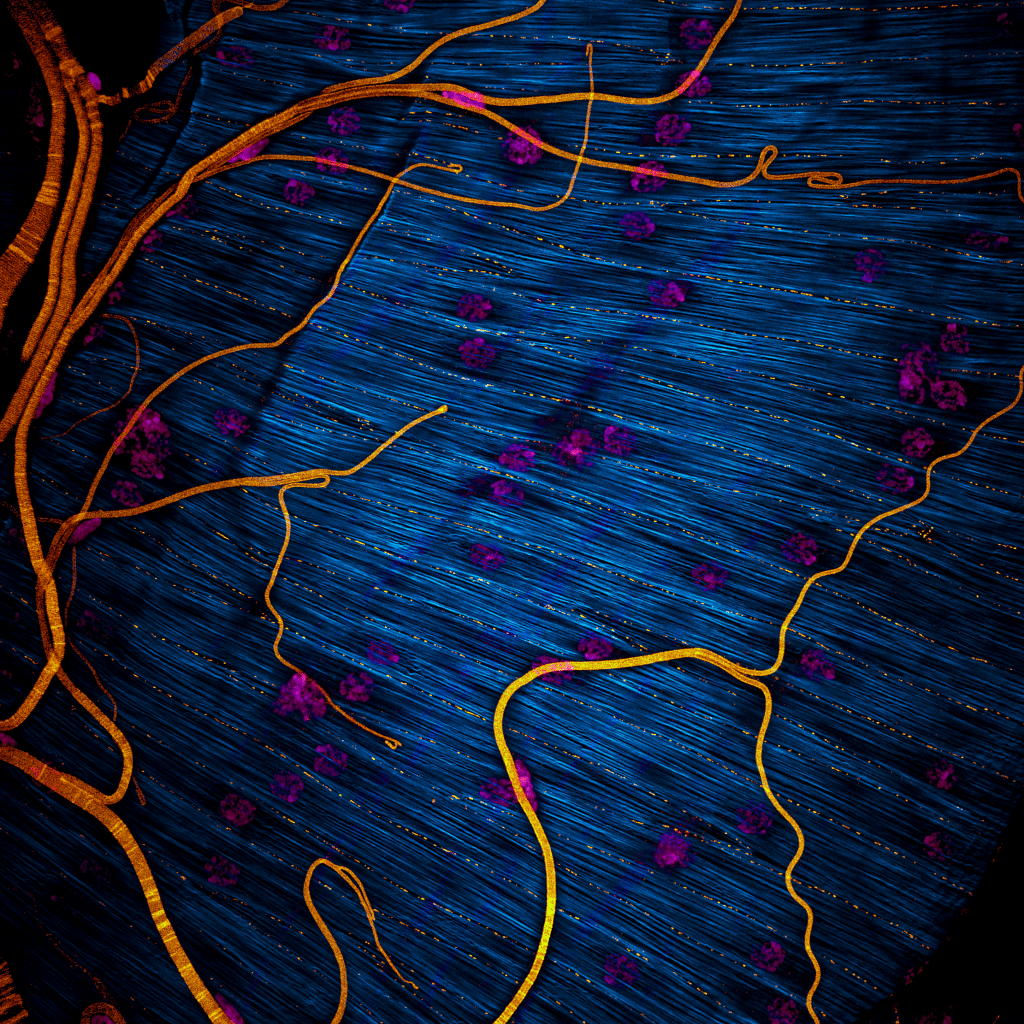
Our featured image, acquired by Maik Bischoff, captures a portion of the testis from the fruit fly Drosophila hydei. Like all insect testes, it is enveloped by a muscle layer. F-actin was stained using phalloidin, making the muscle fibers visible, while DAPI staining revealed the multiple nuclei within each syncytial myotube. An antibody against Drosophila melanogaster N-Cadherin marks the focal cell-cell adhesions that maintain the integrity of the muscle sheet. As a nice side effect, trachea were visible too (probably due to autofluorescence or unspecific antibody binding).
I titled the image ‘Invisible Architects’, inspired by our discovery that in D. melanogaster, it is this very muscle layer that sculpts the testis into its striking, left-right asymmetric spiral shape during development. Intriguingly, different fly species exhibit dramatic variation in the number of spiral turns – from a slight bend to dozens or even hundreds of revolutions. D. hydei is among the species with the most extreme spiraling.
Discover more about Maik’s research
Research career so far: I began my scientific career at the University of Gießen in Germany, where I was introduced to fly genetics and muscle development by Anne Holz during my Master’s studies. I then pursued my PhD at the University of Marburg, working jointly in the labs of Renate Renkawitz-Pohl and Sven Bogdan. During my PhD, I established the fly testis as a novel live-cell imaging model to study collective cell migration of the myotubes that eventually cover and sculpt the testis. As the only researcher working on this unique live-cell imaging model, I was eager to continue. I secured independent funding and moved with my young family to North Carolina to begin a postdoctoral position in Mark Peifer’s lab at UNC Chapel Hill.
Mark has been the best mentor I could have hoped for, supporting my independence while offering valuable guidance. He even secured the help of two talented Postbacs, Jenna Norton and Sarah Clark, who contributed significantly to the progress of my projects. Together, we successfully completed and published two major studies (details in the following paragraph). I also had the opportunity to present our work at international conferences – personal highlights include speaking at the ASCB meeting in San Diego and the Cell Migration Gordon Research Conference in Lucca, Italy.
Current research: As mentioned earlier, I study the Drosophila testis, which becomes covered and sculpted by highly motile muscle cell precursors. This system offers a powerful window into what I believe is an underexplored morphogenetic mechanism: mesenchymal organ sculpting. While much is known about how epithelial tissues bend and fold through mechanisms like apical constriction, mesenchymal cells can also generate complex organ shapes. The Drosophila testis provides a unique model to study such processes.
During my PhD, I focused on the early phase of this process—when myotubes collectively migrate to cover the testis, prior to spiralization. I found that this migration is self-regulated through contact-dependent modulation of integrin adhesion dynamics, creating a self-expanding cell sheet. Interestingly, we observed that cells remain highly protrusive, even at sites of cell-cell contact. My postdoc research highlighted a key role for the classical axon guidance receptor Plexin A in this context. We found that Plexin A activity promotes protrusiveness at junctions – its overactivation drives a hyper-mesenchymal state in which cells lose cohesion, while its inhibition transforms cell-cell edges to look more like epithelial contacts, limiting cell spreading and compromising coverage.
Looking ahead, I plan to establish my own lab to study not only the initial migratory phase but also the later spiralization phase of testis morphogenesis. As a foundation for this work, we recently completed an RNAseq-guided RNAi screen that identified numerous candidate regulators of testis sculpting. Once I set up my lab, I am eager to follow up on these leads and continue uncovering the cell biological principles that drive mesenchymal morphogenesis.
Favourite imaging technique/microscope: My go-to imaging techniques are spinning disk microscopy for long-term live-cell imaging, and confocal Airyscan microscopy using the Zeiss LSM 980, which I primarily use for high-resolution short-term imaging and capturing stills of both live and fixed tissues. These methods provide the temporal and spatial resolution that I need to studying dynamic cellular processes in developing tissues.
What are you most excited about in microscopy? I find label-free imaging techniques, such as holotomography, particularly fascinating. During a holotomography microscope demo at UNC, I was genuinely impressed by how rapidly it could capture cells at subcellular resolution, rendering organelles visible in 3D without the need for any labeling. The ability to visualize cellular architecture in such detail – while preserving native cell states – is incredibly powerful and opens exciting avenues for live-cell imaging.


 (2 votes, average: 1.00 out of 1)
(2 votes, average: 1.00 out of 1)