Featured image with Anthony Dornan and Fara van der Schans
Posted by FocalPlane, on 28 February 2025
Our featured image, acquired by Anthony Dornan and Fara van der Schans, showcases the perinuclear distribution of mitochondria in the highly respiratory human pancreatic ductal adenocarcinoma (PDAC) tumour cells (Panc-1) stained with the mitochondrial stain TMRE (thermal LUT), the mitochondria are associated with the nuclei, stained with DAPI (cyan). The live cell image is a maximum intensity Z-projection generated using a Nikon AXR NSPARC super-resolution microscope and then processed using ImageJ and Photoshop.
While the image readily illustrates the variety of shapes and sizes that comprise the population of mitochondria within the cell, it cannot communicate the dynamic nature of the mitochondria as they rapidly move, fuse and split, but this can be captured using the resonant scanner of the Nikon microscope.
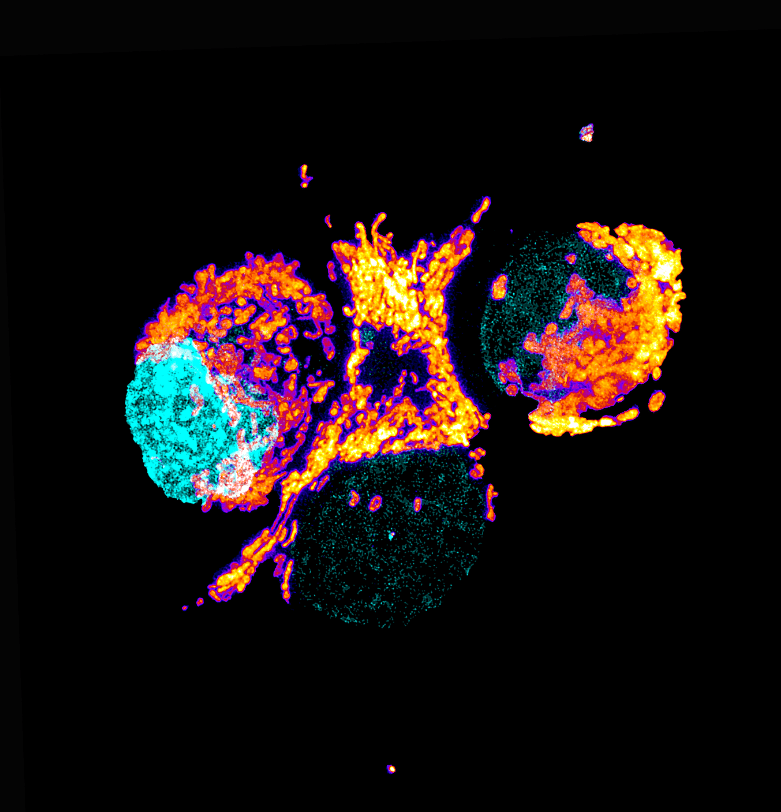
Discover more about Anthony’s and Fara’s research:
Research career so far:
Anthony Dornan: In my final year as an undergraduate in Molecular Biology at the University of Glasgow, I was lucky enough to get a summer internship in the Department of Genetics in the lab of Professor Kim Kaiser, where I was introduced to Drosophila melanogaster, and the vast array of powerful molecular techniques that can be applied to this model organism. Subsequently I was hired to as lab manager/research technician for Dr Stephen Goodwin, where we continued to use Drosophila to study the underlying genetics specifying dimorphic neural circuitry using courtship behaviour as a paradigm. Following the Goodwin Lab’s departure to Oxford, I remained at Glasgow and transferred to the Dow Lab in the School of Molecular Biosciences, where we again used Drosophila, this time studying Malpighian (renal) tubules to investigate osmoregulation, immunity and homeostatic function’s within the fly. Throughout my career, my research has required me to employ, and become expert in, a variety of bioimaging techniques to the extent that I became coordinator of BioImaging for the School of Molecular Biosciences. This expertise allowed me to transfer to my new position as Deputy Lead of the Cellular Analysis Facility – part of the Shared Research Facilities for the College of Medical Veterinary and Life Sciences at the University of Glasgow.
Fara van der Schans: During my MSci Biochemistry at the University of Glasgow, I was lucky to get a summer internship in the Lewis lab at the Scotland Cancer Research UK Institute (formerly the CRUK Beatson Institute). This exposed me to a range of molecular and cellular techniques and confirmed my desire to pursue a career in research. I took a year out to join AstraZeneca as an industrial placement student, where I worked on characterising epidermal growth factor receptor (EGFR) mutants and exploring the mechanisms of EGFR tyrosine kinase inhibitors. In my final year of my undergraduate degree, I undertook an 8-week project in the Tokatlidis lab, investigating protein targeting to mitochondria under oxidative stress, which I continued after graduation through a Lister Institute Summer Studentship. I then spent two years at the Francis Crick Institute studying the role of protein phosphatases in autophagosome formation in budding yeast. Following this, I returned to the University of Glasgow and the Tokatlidis lab to start my doctoral research as a James McCune Smith PhD scholar.
Current research:
AD: My role is now one of expert support, ensuring the Cellular Analysis imaging facility, and its constituent imaging platforms, runs smoothly, while training and supporting researchers and research groups to pursue their imaging needs. The featured image is a case in point, with Fara providing the stained cell lines and while I supported her to generate images and data from her samples.
FvdS: My doctoral research addresses the clinical challenge of eradicating therapy-resistant pancreatic cancer cells. With pancreatic cancer projected to become the second leading cause of cancer-related deaths by 2030, treatment options remain severely limited by chemoresistance. Therapy-resistant cancer cells often depend on mitochondrial function; therefore, my work investigates the role of mitochondrial biogenesis in promoting pancreatic cancer progression and therapy resistance. To tackle this problem, we’re using a wide range of techniques, including biochemical, biophysical, cellular, and multi-omics approaches. This multi-disciplinary approach not only enriches my research but also enables collaborations with expert research facilities, both within the University of Glasgow – such as the Cellular Analysis Imaging Facility – and with external groups.
Favourite imaging technique/microscope:
AD: As the majority of my research/imaging career has involved realising fluorescence images using various confocal systems (Zeiss, Nikon and Leica mainly) it would be disloyal to highlight any other form of microscopy. That said each of the diverse techniques and systems I have used throughout my career, from DIC/Phase Contrast, to fluorescence imaging, to SEM and TEM, to SIM Lattice, to Mesolens and Lightsheet etc. has immediately captivated me and filled me with wonder at being able to visualise gene expression and the biological processes that direct living systems.
FvdS: As a biochemist by training, diving into imaging techniques – with the help of Tony (Anthony) and the Cellular Analysis Facilities team – has been an exciting new challenge. For my project, we’re exploring mitochondrial networks and dynamics in pancreatic cancer cells at high resolution. Using the Nikon AXR NSPARC super-resolution confocal microscope has been incredible so far and I’ve captured some great images. In future, I look forward to using electron microscopy techniques for further insights.
What are you most excited about in microscopy?
AD: I think one of the most exciting advances in microscopy as a whole is how disparate techniques are being joined together to form imaging pipelines. This means that it is possible to move from the macro, using techniques such as mesolenses or lightsheet technologies to image whole animal or tissues, down to the molecular, where we can map 3D cellular architecture using advanced EM techniques, to single-molecules and beyond. I’m also excited about incorporating 4D components and live cell imaging to be able to delineate functional biological processes in real-time.
FvdS: What excites me most about microscopy is its ability to reveal molecular structures and processes in real time with incredible detail. It enables me to tackle scientific questions from unique angles that wouldn’t be possible otherwise. A technique that particularly excites me is cryo-electron tomography which allows one to visualise mitochondria in 3D at high resolution whilst preserving their native state.


 (No Ratings Yet)
(No Ratings Yet)