Featured image with Christina Daly
Posted by FocalPlane, on 6 January 2026
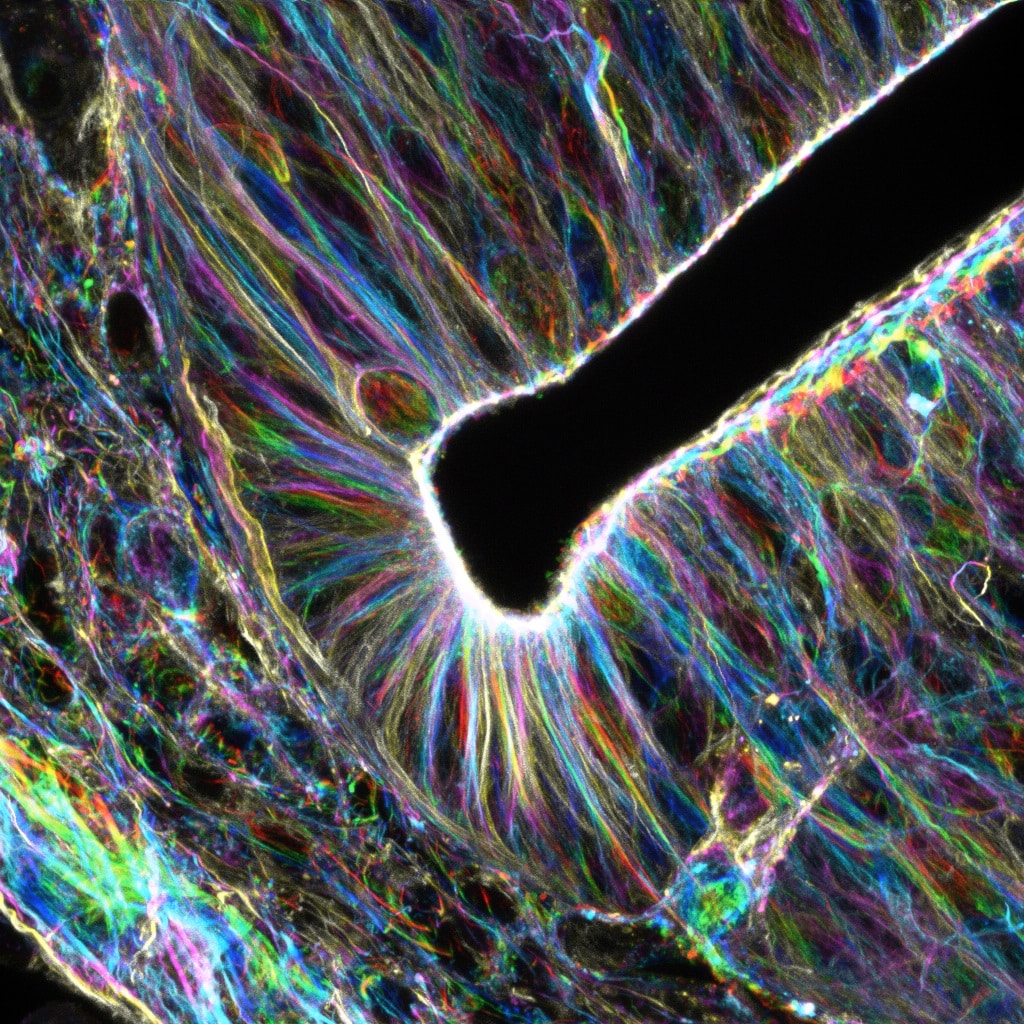
Our featured image, acquired by Christina Daly, is of an E9.5 murine neural tube. The embryo was fixed in 4% Paraformaldehyde, embedded in agarose, and vibratome-sectioned at 100 mm thickness. The sections were stained with for F-actin with phalloidin and imaged on a Nikon A1R confocal. Z stacks were acquired with a 60X objective and temporally color-coded in FIJI/ImageJ for Z-depth shading.
Discover more about Christina’s research.
Research career so far: I started my research journey as an undergraduate at Appalachian State University, where I first used confocal microscopy to help confirm Vibrio cholerae infection of a zebrafish model organism for my honors thesis research in the labs of Drs. Ted Zerucha and Ece Karatan (2013). This introduction to microscopy led me to earn my master’s degree (2016) in Dr. Darren Seals’ lab, where I studied actin cytoskeletal regulation in invading prostate cancer cells. Following my master’s, I spent a few years at Wake Forest University investigating B cell immunology in immunization, infection, aging, and anti-tumor immunity under the mentorship of Dr. Karen Haas. My varied experiences in the actin cytoskeleton and advanced tissue handling techniques brought me to the PhD program at St. Jude Children’s Research Hospital in 2018. I worked under Dr. Stacey Ogden on actin regulation and ligand transport in cultured cells, earning my PhD in 2024, and have since stayed on as a postdoc to confirm my findings in a murine embryonic model.
Current research: The Ogden lab studies the regulation of the Sonic Hedgehog (SHH) pathway in mammalian development. My personal project has focused on SHH ligand transport, which can be accomplished through extension of incredibly long, thin signaling filopodia called “cytonemes”. Through specialized fixation and processing methods developed by our lab, I was able to visualize these cellular extensions and systematically uncover some of the actin regulatory machinery responsible for inducing cytoneme outgrowth for SHH ligand dissemination. As a postdoc, I have focused on validating this pathway in a mouse embryo model, with a particular focus on neural tube development. Proper development of the neural tube, the presumptive spinal cord, relies on precise concentrations and durations of SHH pathway activation. Therefore, neural tube imaging can provide a sensitive and accurate readout of SHH pathway integrity.
Favourite imaging technique/microscope: Since my research focus is on preserving and imaging delicate extensions in tissues, I am incredibly reliant on techniques that minimize tissue processing and handling. Therefore, I prefer use of genetically-encoded fluorophores, such as Cre-driven membraneTomato-membraneGFP murine models. Additionally, I appreciate the ease of the vibratome, which requires little to no tissue processing for consistent sectioning. While all the microscopes in our microscopy facility have their advantages, I have recently preferred the Nikon A1R for routine fixed and live confocal microscopy due to the intuitive user interface.
What are you most excited about in microscopy? I am aways in awe of the innovation and creativity in the imaging field. In cultured cells, I enjoy the creative tools used to manipulate biological processes or cellular component function/localization to uncover new aspects of cellular biology. It is incredibly rewarding to successfully implement newly developed tools and strategies, and I appreciate the accessibility of these novel approaches made available by creative scientists in the imaging sciences. Since I study intercellular communication in tissue morphogenesis, I am encouraged by the advances we have seen in live imaging of tissues, both in technique and instrumentation. I am hopeful that some of the incredible imaging strategies conducted to study embryogenesis of invertebrates and non-mammalian vertebrates can be extended to mammalian models, which are more challenging due to their larger sizes and more complex tissue topology. These future advancements could help us better understand the conservation of morphogenetic processes between species.


 (1 votes, average: 1.00 out of 1)
(1 votes, average: 1.00 out of 1)
Beautiful. Incredible. Spectacular I love this so much.